3.1 Review of Acids and Bases and Ka
3.1 酸碱和Ka的回顾
The most commonly applied definition of acids and bases is the Brønsted-Lowry definition:
最常用的酸和碱定义是 Brønsted-Lowry 定义:
Brønsted-Lowry Acid: a substance that can donate a proton (H+)
Brønsted-Lowry Acid:一种可以提供质子的物质(H + )Brønsted-Lowry Base: a substance that can accept a proton (H+)
Brønsted-Lowry 碱:可以接受质子的物质 (H + )
Therefore, according to the Brønsted-Lowry definition, an acid-base reaction is a proton transfer process in which the acid gives away a proton and the base accepts a proton as shown in the general equation:
因此,根据 Brønsted-Lowry 定义,酸碱反应是一个质子转移过程,其中酸放出质子,碱接受质子,如通式所示:
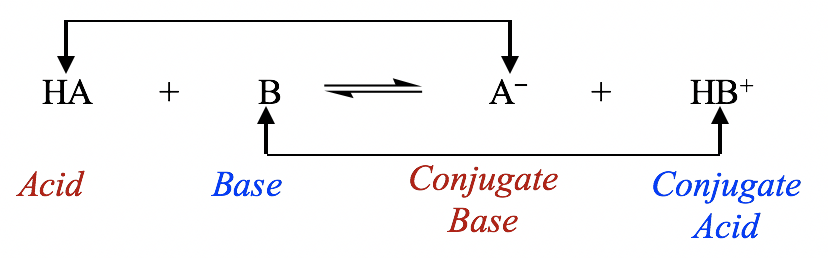
General equation for acid-base reaction
酸碱反应的一般方程
The species that forms when an acid loses its proton is called the conjugate base of that acid; similarly, the species that forms when a base accepts a proton is called the conjugate acid of that base. In the general equation above, HA is the conjugate acid of A–, and A– is the conjugate base of HA. HA and A– can also be called a conjugate acid-base pair; another pair is HB+ and B.
当酸失去质子时形成的物质称为该酸的共轭碱;同样,当碱接受质子时形成的物质称为该碱的共轭酸。在上面的通式中,HA是A – 的共轭酸,A – 是HA的共轭碱。 HA和A – 也可称为共轭酸碱对;另一对是 HB + 和 B。
A strong acid donates the proton completely, and the arrow “→” can be used in the reaction equation to indicate that the reaction goes to completion. The dissociation reaction of the strong acid HCl in water is used as an example here:
强酸完全给出质子,反应方程式中可用箭头“→”表示反应完全。这里以强酸性HCl在水中的解离反应为例:
HCl (g) + H2O (l) →H3O+(aq) + Cl–(aq)
For weak acids (HA is used as a general formula), the proton is only partially donated and the reaction stays at equilibrium. The equilibrium arrow “ ” will be needed in the reaction equation to indicate the equilibrium status:
” will be needed in the reaction equation to indicate the equilibrium status:
对于弱酸(HA用作通式),仅提供部分质子,反应保持平衡。反应方程中需要平衡箭头“  ”来指示平衡状态:
”来指示平衡状态:
HA (aq) + H2O (l) ⇔ H3O+ (aq) + A– (aq)
The equilibrium constant for the above reaction is called the acid dissociation constant, Ka. It is a constant to measure the relative strength of an acid. The expression for Ka is:
上述反应的平衡常数称为酸解离常数,K a. 它是衡量酸相对强度的常数。 K a 的表达式为:
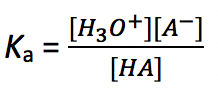
The larger the Ka value, the stronger the ability of the acid to donate protons, and the stronger the acid is. (Technically, when the Ka value is larger than 10, the acid can be regarded as a strong acid.)
K a 值越大,酸贡献质子的能力越强,酸越强。 (从技术上讲,当K a 值大于10时,酸可以被视为强酸。)
For the conjugate acid-base pair, the stronger the acid, the weaker the conjugate base is, and vice versa.
对于共轭酸碱对来说,酸越强,共轭碱越弱,反之亦然。
来源于资源矩阵知识星球【仪器分析学苑】
更多内容看下边:
当你在仪器分析工作的时候:
是否曾为一份技术教程无处找到而发愁? 是否在遇到问题时候无人解答? 是否需要提升认知的时候google不到自己想要的答案? 是否面对日益激烈的分析行业无人分析? 是否想告别那些成天都是广告满天飞的行业社群? 这些问题在我们的知识星球都能解决,这是仪器分析行业(色谱质谱)专属的知识星球,一个属于▶仪器分析◀色谱质谱行业的优质付费社群! 商业的底层逻辑是什么,是最短时间得到最准确的信息,加入知识星球能帮你节约宝贵时间,让你专注学习,分享,交流!让你专注于学习、分享、交流和商业洽谈。通过最低的成本,你可以获得最大的价值。
经过近200个日日夜夜的积累,仪器分析学苑知识星球已经囊括了超过100+份产品技术文档,视频,经验分享,行业报告等等。
还能加入特定交流群得到本人和各位大佬的指导
及其丰富的书籍资源,也可以提供相关的技术咨询

随着资料逐渐增加,会员人数逐渐增多,每隔半年,星球价格会依据情况相应上浮,早加入,早享受,等待就是成本!
星球价格,不过一顿饭,但是星球却可以持续的给你提供价值,助力市场开拓,销售增长,职场提升。还能扩大行业链接,增加创富可能!
目前扫码就可以加入:
知识星球1:仪器分析学家的培养基地:仪器分析学苑

知识星球2:只需要某某元,一顿茶水钱,扫码有惊喜,医药投资、了解医药创业,投资,职业的平台,附带群资源

vx:加我微信咨询:关注公众号-我的-联系我们,扫码加
医药仪器创业方面的群聊:邀请制或者加入知识星球 总结不易,大佬肯赞赏否? 
END
声明:本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
垂直文章