3.2 Organic Acids and Bases and Organic Reaction Mechanism
3.2 有机酸、有机碱及有机反应机理
3.2.1 Organic Acids 3.2.1 有机酸
The acids we discussed in general chemistry usually refer to inorganic acids, such as HCl, H2SO4, and HF. If the structure of the acid contains a “carbon” part, then it is an organic acid. Organic acids donate protons in the same way as inorganic acids, but their structure may be more complicated due to the nature of organic structures.
我们在普通化学中讨论的酸通常是指无机酸,例如HCl、H 2 SO 4 和HF。如果酸的结构含有“碳”部分,则它是有机酸。有机酸以与无机酸相同的方式提供质子,但由于有机结构的性质,它们的结构可能更复杂。
Carboxylic acid, with the general formula of R-COOH, is the most common organic acid we are familiar with. Acetic acid (CH3COOH), an ingredient in vinegar, is a simple example of a carboxylic acid. The Ka of acetic acid is 1.8×10-5.
羧酸,通式为R-COOH,是我们最熟悉的有机酸。醋酸 (CH 3 COOH) 是醋中的一种成分,是羧酸的一个简单例子。乙酸的K a 为1.8×10 -5 。
Another common organic acid is the organic derivative of sulfuric acid H2SO4.
另一种常见的有机酸是硫酸的有机衍生物H 2 SO 4 。
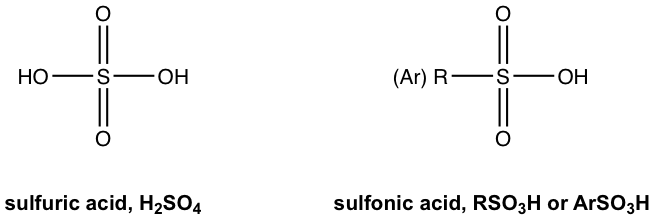
The replacement of one OH group in H2SO4 with a carbon-containing R (alkyl) or Ar (aromatic) group leads to the organic acid named “sulfonic acid” with the general formula of RSO3H, or ArSO3H. Sulfonic acid is a strong organic acid with a Ka in the range of 106. The structure of a specific sulfonic acid example called p-toluenesulfonic acid is shown here:
用含碳的 R(烷基)或 Ar(芳香族)基团取代 H 2 SO 4 中的一个 OH 基团,得到称为“磺酸”的有机酸,其结构式为:通式为RSO 3 H,或ArSO 3 H。磺酸是一种强有机酸,K a 在10 6
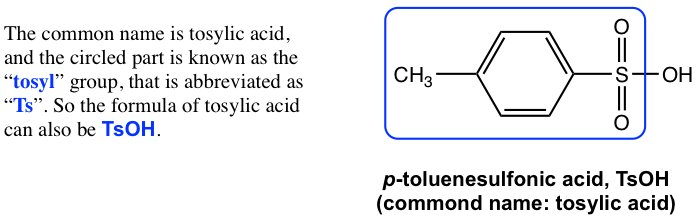
Figure 3.1a CH3C6H4SO3H Tosylic acid
图 3.1a CH3C6H4SO3H 甲苯磺酸
Other than the acids mentioned here, technically, any organic compound could be an acid because organic compounds always have hydrogen atoms that could potentially be donated as H+. A few examples are shown here with the hydrogen atoms highlighted in blue:
从技术上讲,除了这里提到的酸之外,任何有机化合物都可以是酸,因为有机化合物总是含有氢原子,有可能以 H+ 的形式提供。这里显示了一些示例,其中氢原子以蓝色突出显示:

Therefore, the scope of acids has been extended to be broadly extended in an organic chemistry context. We will have further discussions on the acidity of organic compounds in section 3.3, and we will see more acid-base reactions applied to organic compounds later in this chapter.
因此,酸的范围已扩展到有机化学领域。我们将在 3.3 节中进一步讨论有机化合物的酸性,并且在本章后面我们将看到更多应用于有机化合物的酸碱反应。
3.2.2 Organic Bases 3.2.2 有机碱
虽然由于总是涉及氢原子,识别有机酸相对简单,但识别有机碱并不总是容易。根据定义,碱基是能够接受质子的物质。有机碱可能涉及各种不同的结构,但它们必须具有共同的特征,即具有能够接受质子的电子对。电子对可以是中性或带负电物质上的孤对电子或π电子对。因此,有机碱可能涉及以下类型:
Negatively charged organic bases: RO–(alkyloxide), RNH–(amide), and R–(alkide, the conjugate base of alkane). Since the negatively charged bases have a high electron density, they are usually stronger bases than the neutral ones.
带负电荷的有机碱:RO-(烷基氧化物)、RNH-(酰胺)和R-(醇盐,烷烃的共轭碱)。由于带负电的碱基具有高电子密度,因此它们通常比中性碱基更强。
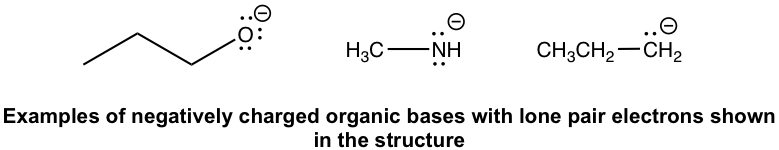
Note: Keep in mind that lone pairs are usually omittedin organic structures as mentioned before. For example, with the formula of CH3NH– given, you should understand that the N actually has two pairs of lone pair electrons (as shown in the above structure) and it is a base.
注意:请记住,如前所述,有机结构中通常会省略孤对电子对。例如,给出CH 3 NH – 的公式,你应该明白N实际上有两对孤对电子(如上面的结构所示),它是一个基地。
Neutral organic bases, for example amine, C=O group and C=C group
中性有机碱,例如胺、C=O基团和C=C基团
Amine: RNH2, R2NH, R3N, ArNH2, etc. (section 2.3). As organic derivatives of NH3, which is an inorganic weak base, amines are organic weak bases with lone pair electrons on N that are able to accept the protons.
胺:RNH 2 、R 2 NH、R 3 N、ArNH 2, 等(2.3节)。作为无机弱碱NH 3 的有机衍生物,胺是N上具有能够接受质子的孤对电子的有机弱碱。
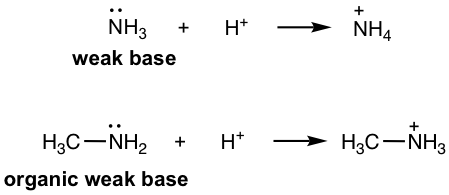
Figure 3.1b weak base and organic weak base
图3.1b 弱碱和有机弱碱
Functional groups containing oxygen atoms: carbonyl group C=O, alcohol R-OH, and ether R-O-R. The lone pair electrons on O in these groups are able to accept the protons, so functional groups like aldehyde, ketone, alcohol and ether are all organic bases. It may not be easy to accept this concept at first because these groups do not really look like bases. However, they are bases according to the definition because they are able to accept the proton with the lone pair on the oxygen atom.
含氧原子的官能团:羰基C=O、醇R-OH、醚R-O-R。这些基团中O上的孤对电子能够接受质子,因此醛、酮、醇、醚等官能团都是有机碱。一开始接受这个概念可能并不容易,因为这些团体看起来并不像基地。然而,根据定义,它们是碱,因为它们能够接受氧原子上具有孤对电子的质子。
Adjust your thinking here to embrace thebroader scopeof acids and bases in an organic chemistry context.
在此调整您的思维,以涵盖有机化学背景下更广泛的酸和碱范围。
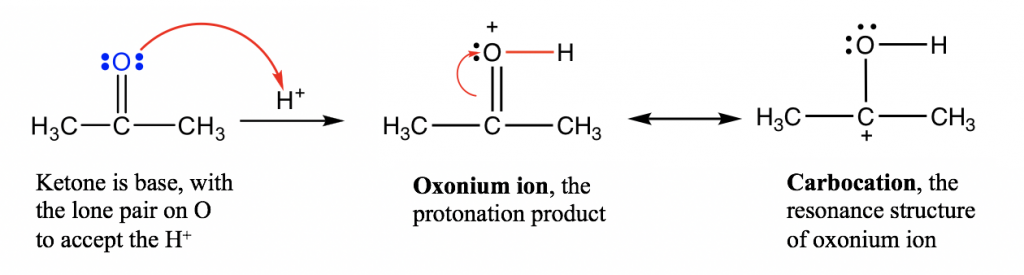
Here, we will take the reaction between acetone and H+ as an example, to understand the reaction deeply by exploring the reaction mechanism,and learn how to use the curved arrows to show it.
在这里,我们以丙酮与H + 的反应为例,通过探索反应机理来深入理解该反应,并学习如何使用弯曲箭头来表示。
A reaction mechanismis the step-by-step electron transfer process that converts reactants to products. Curved arrowsare used to illustrate the reaction mechanism. Curved arrows should always start at the electrons, and end in the spot that is receiving the electrons. The curved arrows used here are similar to those for resonance structures (section 1.4) but are not exactly the same though. Please note that in resonance structures, curved arrows are used to show how the electrons are transferred withinthe molecule, leading to another resonance structure. For mechanism purposes, there must be arrows that connect between species.
反应机理是将反应物转化为产物的逐步电子转移过程。弯曲箭头用于说明反应机理。弯曲箭头应始终从电子开始,并在接收电子的点结束。这里使用的弯曲箭头与共振结构(第 1.4 节)的弯曲箭头相似,但并不完全相同。请注意,在共振结构中,弯曲箭头用于显示电子如何在分子内转移,从而形成另一个共振结构。出于机制目的,物种之间必须有连接的箭头。
Notes for the above mechanism:
上述机制的注意事项:
For the acid-base reaction between the C=O group and the proton, the arrow starts from the electron pair on O and points to the H+ that is receiving the electron pair. A new O-H bond is formed as a result of this electron pair movement.
对于 C=O 基团和质子之间的酸碱反应,箭头从 O 上的电子对开始,指向接收电子对的 H + 。由于电子对运动,形成了新的 O-H 键。In this acid-base reaction, ketone is protonated by H+ so this reaction can also be called the “protonation of ketone”.
在这个酸碱反应中,酮被H + 质子化,所以这个反应也可以称为“酮的质子化”。The product of the protonation is called an “oxonium ion”, which is stabilized with another resonance structure, carbocation.
质子化的产物称为“氧鎓离子”,它通过另一种共振结构碳正离子来稳定。
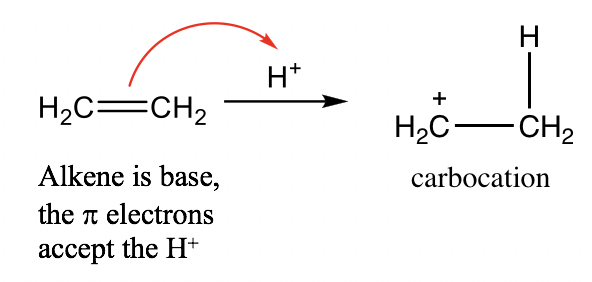
Alkene (C=C): Although there are no lone pair electrons in the C=C bond of alkene, the π electrons of the C=C double bond are able to accept protons and acts as a base. For example:
烯烃(C=C):虽然烯烃的C=C键中没有孤对电子,但C=C双键的π电子能够接受质子并充当碱基。例如:
Example: Organic acid and base reaction
示例:有机酸和碱反应
Predict and draw the products of following reaction and use curved arrow to show the mechanism.
预测并画出下列反应的产物,并用曲线箭头表示其机理。
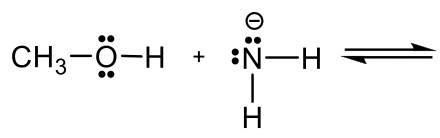
Approach: If H+ is the acid as in previous examples, it is rather easy to predict how the reaction will proceed. However, if there is no obvious acid (or base) as in this example, how do you determine which is the acid, and which is the base?
方法:如果 H + 是前面例子中的酸,则很容易预测反应将如何进行。但是,如果像本例一样没有明显的酸(或碱),那么如何确定哪个是酸,哪个是碱?
Methanol CH3OH is neutral, and the other reactant, NH2–, is a negatively charged amide. The amide with a negative charge has higher electron density than the neutral methanol, therefore amide NH2–should act as base, and CH3OH is the acid that donates H+.
甲醇CH 3 OH 是中性的,另一个反应物NH 2 – 是带负电的酰胺。带负电荷的酰胺具有比中性甲醇更高的电子密度,因此酰胺 NH 2 – 应充当碱,CH 3 OH 为酸捐赠 H + 。
Solution:解决方案:
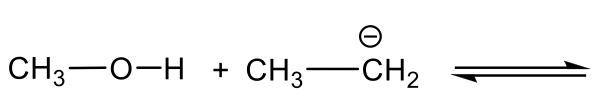
来源于资源矩阵知识星球【仪器分析学苑】
更多内容看下边:
当你在仪器分析工作的时候:
是否曾为一份技术教程无处找到而发愁? 是否在遇到问题时候无人解答? 是否需要提升认知的时候google不到自己想要的答案? 是否面对日益激烈的分析行业无人分析? 是否想告别那些成天都是广告满天飞的行业社群? 这些问题在我们的知识星球都能解决,这是仪器分析行业(色谱质谱)专属的知识星球,一个属于▶仪器分析◀色谱质谱行业的优质付费社群! 商业的底层逻辑是什么,是最短时间得到最准确的信息,加入知识星球能帮你节约宝贵时间,让你专注学习,分享,交流!让你专注于学习、分享、交流和商业洽谈。通过最低的成本,你可以获得最大的价值。
经过近200个日日夜夜的积累,仪器分析学苑知识星球已经囊括了超过100+份产品技术文档,视频,经验分享,行业报告等等。
还能加入特定交流群得到本人和各位大佬的指导
及其丰富的书籍资源,也可以提供相关的技术咨询

随着资料逐渐增加,会员人数逐渐增多,每隔半年,星球价格会依据情况相应上浮,早加入,早享受,等待就是成本!
星球价格,不过一顿饭,但是星球却可以持续的给你提供价值,助力市场开拓,销售增长,职场提升。还能扩大行业链接,增加创富可能!
目前扫码就可以加入:
知识星球1:仪器分析学家的培养基地:仪器分析学苑

知识星球2:只需要某某元,一顿茶水钱,扫码有惊喜,医药投资、了解医药创业,投资,职业的平台,附带群资源

vx:加我微信咨询:关注公众号-我的-联系我们,扫码加
医药仪器创业方面的群聊:邀请制或者加入知识星球 总结不易,大佬肯赞赏否? END
声明:本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
垂直文章