3.4.1 Element Effect 3.4.1 元素影响
A. Periodic Trend: Electronegativity
A. 周期性趋势:电负性
The element effect is about the individual atom that connects with the hydrogen (keep in mind that acidity is about the ability to donate a certain hydrogen). Let’s compare the acidity of hydrogens in ethane, methylamine and ethanol as shown below.
元素效应与与氢连接的单个原子有关(请记住,酸度与提供特定氢的能力有关)。让我们比较一下乙烷、甲胺和乙醇中氢的酸性,如下所示。
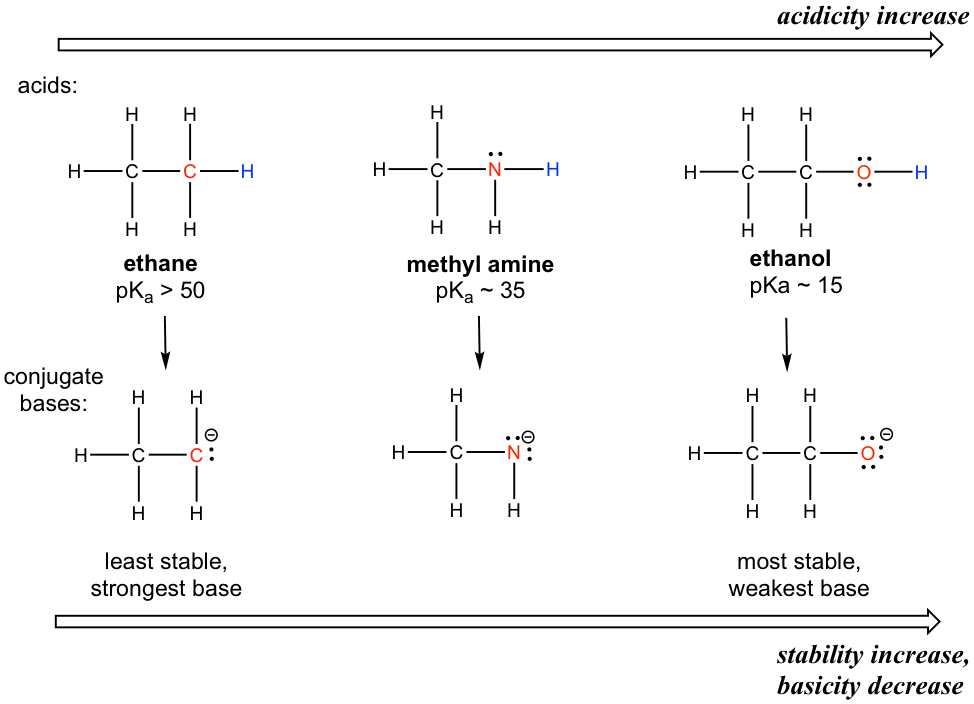
A clear trend in the acidity of these compounds is that the acidity increases for the elements from left to right along the second row of the periodic table, C to N, and then to O. This is consistent with the increasing trend of EN along the period from left to right. The connection between EN and acidity can be explained as the atom with a higher EN being better able to accommodate the negative charge of the conjugate base, thereby stabilizing the conjugate base in a better way. Therefore, the more stable the conjugate base, the weaker the conjugate base is, and the stronger the acid is. For the discussion in this section, the trend in the stability (or basicity) of the conjugate bases often helps explain the trend of the acidity.
这些化合物酸度的一个明显趋势是,元素周期表第二行从左到右,C到N,然后到O的元素的酸度增加。这与EN沿着元素周期表的增加趋势一致。期间从左到右。 EN与酸度之间的联系可以解释为EN较高的原子能够更好地容纳共轭碱的负电荷,从而更好地稳定共轭碱。因此,共轭碱越稳定,共轭碱越弱,酸性越强。对于本节的讨论,共轭碱的稳定性(或碱性)趋势通常有助于解释酸性趋势。
The relative acidity of elements in the same period is:
同期元素的相对酸度为:
For elements in the same period, the more electronegative an atom, the stronger the acid is; the acidity increases from left to right across the period.
对于同一周期的元素,原子电负性越大,酸越强;在此期间,酸度从左到右逐渐增加。
B. Group (vertical) Trend: Size of the atom
B. 组(垂直)趋势:原子大小
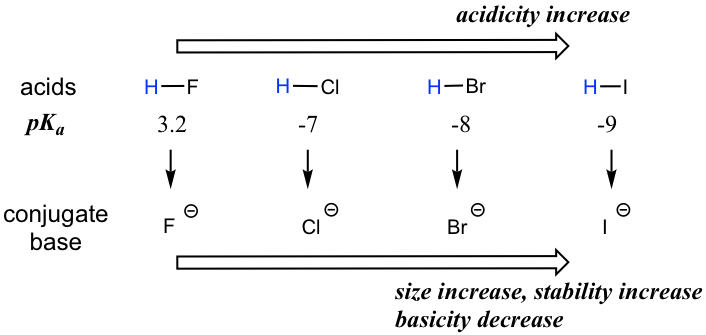
When moving vertically within a given group on the periodic table, the trend is that acidity increases from top to bottom. This can be illustrated with the haloacids HX and halides as shown below: the acidity of HX increases from top to bottom, and the basicity of the conjugate bases X– decreases from top to bottom.
当在元素周期表上的给定族内垂直移动时,趋势是酸度从上到下增加。这可以用卤代酸HX和卤化物来说明,如下所示:HX的酸性从上到下增加,共轭碱X – 的碱性从上到下减少。
The acidity of the H in thiol SH group is also stronger than the corresponding alcohol OH group following the same trend. For example, the pKa of CH3CH2SH is ~10, which is much more acidic than ethanol CH3CH2OH which has a pKa of ~16.
硫醇SH基团中H的酸性也比相应的醇OH基团强,遵循相同的趋势。例如,CH 3 CH 2 SH 的 pK a 约为 10,其酸性比乙醇 CH 3 CH 强得多 2 OH,其 pK a 约为 16。
To make sense of this trend, we will once again consider the stability of the conjugate bases. When moving vertically in the same group of the periodic table, the size of the atom overrides its EN with regard to basicity. The atomic radius of iodine is approximately twice that of fluorine, so in an iodide ion, the negative charge is spread out over a significantly larger volume, so I– is more stable and less basic, making HI more acidic.
为了理解这一趋势,我们将再次考虑共轭碱基的稳定性。当在元素周期表的同一族中垂直移动时,原子的大小在碱度方面覆盖其 EN。碘的原子半径大约是氟的两倍,因此在碘离子中,负电荷分布在更大的体积上,因此 I– 更稳定且碱性更低,使 HI 更具酸性。
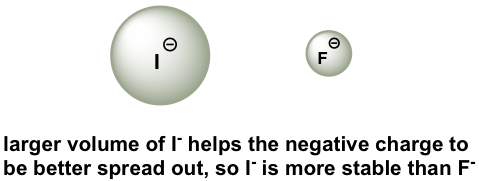
Figure 3.4a Stability of fluorine and iodide ion
图3.4a 氟离子和碘离子的稳定性
The relative acidity of elements in the same group is:
同族元素的相对酸度为:
For elements in the same group, the larger the size of the atom, the stronger the acid is; the acidity increases from top to bottom along the group.
同族元素,原子尺寸越大,酸性越强;酸度沿组从上到下逐渐增加。
3.4.2. Resonance Effect 3.4.2.共振效应
The resonance effect accounts for the acidity difference between ethanol and acetic acid. For both ethanol and acetic acid, the hydrogen is bonded with the oxygen atom, so there is no element effect that matters. However, the pKa values (and the acidity) of ethanol and acetic acid are very different. What makes a carboxylic acid so much more acidic than an alcohol. As stated before, we begin by considering the stability of the conjugate bases, remembering that a more stable (weaker) conjugate base corresponds to a stronger acid.
共振效应解释了乙醇和乙酸之间的酸度差异。对于乙醇和乙酸来说,氢与氧原子键合,因此不存在重要的元素效应。然而,乙醇和乙酸的 pK a 值(和酸度)非常不同。是什么使羧酸比醇的酸性大得多。如前所述,我们首先考虑共轭碱的稳定性,记住更稳定(更弱)的共轭碱对应于更强的酸。
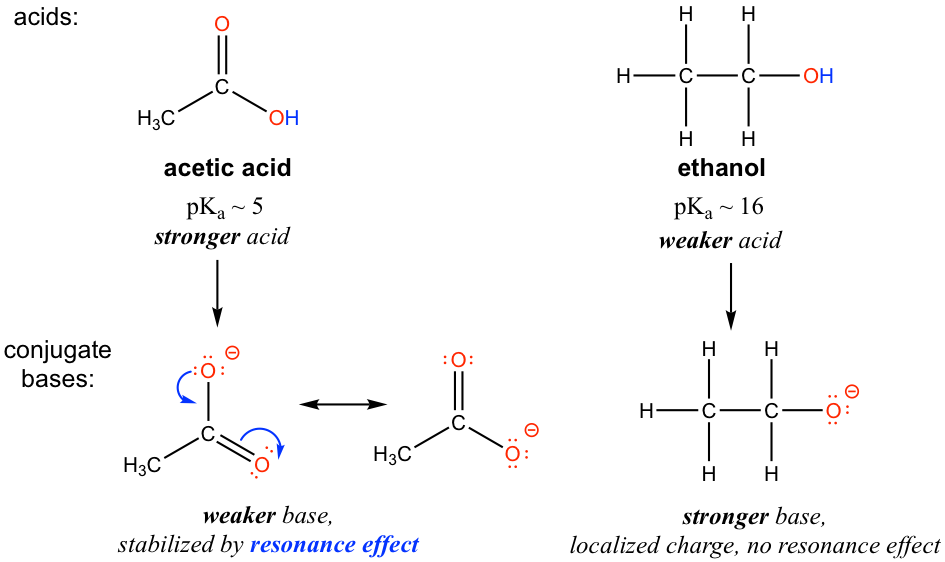
For acetate, the conjugate base of acetic acid, two resonance contributors can be drawn and therefore the negative charge can be delocalized (shared) over two oxygen atoms. However, no other resonance contributor is available in the ethoxide ion, the conjugate base of ethanol, so the negative charge is localized on the oxygen atom. As we have learned in section 1.3, the species that has more resonance contributors gains stability; therefore acetate is more stable than ethoxide and is weaker as the base, so acetic acid is a stronger acid than ethanol.
对于乙酸盐(乙酸的共轭碱),可以绘制两个共振贡献者,因此负电荷可以在两个氧原子上离域(共享)。然而,乙醇离子(乙醇的共轭碱)中没有其他共振贡献者,因此负电荷位于氧原子上。正如我们在 1.3 节中了解到的,具有更多共振贡献者的物种获得稳定性;因此乙酸比乙醇盐更稳定,并且作为碱更弱,因此乙酸是比乙醇更强的酸。
The charge delocalization by resonance has a powerful effect on the reactivity of organic molecules, enough to account for the significant difference of over 10 pKa units between ethanol and acetic acid. pKa = –log Ka, which means that there is a factor of about 1010 between the Ka values for the two molecules!
共振引起的电荷离域对有机分子的反应性具有强大的影响,足以解释乙醇和乙酸之间超过 10 pK a 单位的显着差异。 pK a = –log K a ,这意味着 K a 值之间存在大约 10 10 的因子两个分子!
Examples 例子
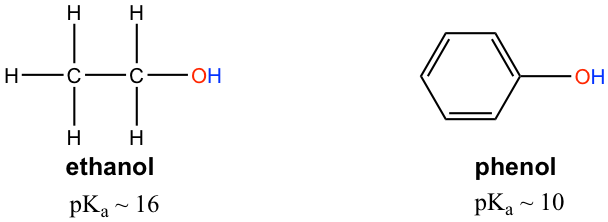
The pKa of the OH group in alcohol is about 15, however OH in phenol (OH group connected on a benzene ring) has a pKa of about 10, which is much stronger in acidity than other alcohols. Explain the difference.
醇中 OH 基团的 pK a 约为 15,而苯酚中的 OH(连接在苯环上的 OH 基团)的 pK a 约为 10,这要强得多酸度高于其他醇。解释一下区别。
Solution: 解决方案:
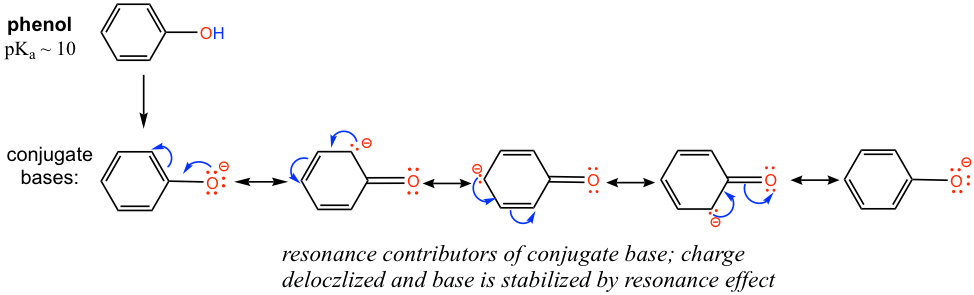
The difference can be explained by the resonance effect. There is no resonance effect on the conjugate base of ethanol, as mentioned before. However, the conjugate base of phenol is stabilized by the resonance effect with four more resonance contributors, and the negative is delocalized on the benzene ring, so the conjugate base of phenol is much more stable and is a weaker base. Therefore phenol is much more acidic than other alcohols.
这种差异可以用共振效应来解释。如前所述,乙醇的共轭碱不存在共振效应。然而,苯酚的共轭碱通过四个共振贡献者的共振效应而稳定,并且负电离域在苯环上,因此苯酚的共轭碱更加稳定并且是较弱的碱。因此,苯酚的酸性比其他醇强得多。
Exercises 3.2 练习3.2
Practice drawing the resonance structures of the conjugate base of phenol by yourself!
自己练习画出苯酚共轭碱的共振结构!It is because of the special acidity of phenol (and other aromatic alcohols), that NaOH can be used to deprotonate phenol effectively, but not to normal alcohols, like ethanol. Show the reaction equations of these reactions and explain the difference by applying the pKa values.
正是由于苯酚(和其他芳香醇)的特殊酸性,NaOH可以有效地使苯酚去质子化,但不能使乙醇等普通醇去质子化。显示这些反应的反应方程,并通过应用 pK a 值来解释差异。
Answers to Chapter 3 Practice Questions第 3 章练习题答案
3.4.3 Inductive Effect 3.4.3 感应效应
Let’s compare the pKa values of acetic acid and its mono-, di-, and tri-chlorinated derivatives:
让我们比较一下乙酸及其单、二和三氯化衍生物的 pK a 值:
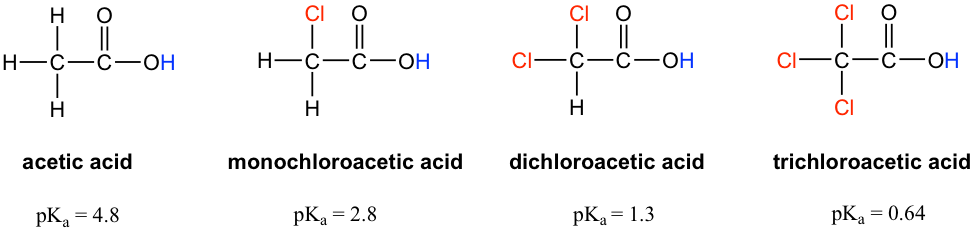
Figure 3.4b Acetic acid and its mono-, di-, and tri-chlorinated derivatives
图 3.4b 乙酸及其单、二、三氯化衍生物
The presence of the chlorine atoms clearly increases the acidity of the carboxylic acid group, and the trending here apparently can not be explained by the element effect. The resonance effect does not apply here either, because no additional resonance contributors can be drawn for the chlorinated molecules. Rather, the explanation for this phenomenon involves something called the inductive effect. A chlorine atom is more electronegative than hydrogen and is thus able to ‘induce’ or ‘pull’ electron density towards itself via σ bonds in between, and therefore it helps spread out the electron density of the conjugate base, the carboxylate, and stabilize it. The chlorine substituent can be referred to as an electron withdrawing group because of the inductive effect.
氯原子的存在明显增加了羧酸基团的酸性,并且这里的趋势显然不能用元素效应来解释。共振效应也不适用于此,因为无法为氯化分子得出额外的共振贡献者。相反,对这种现象的解释涉及一种称为归纳效应的东西。氯原子比氢更具负电性,因此能够通过其间的 σ 键“诱导”或“拉动”电子密度,从而有助于分散共轭碱、羧酸盐的电子密度并使其稳定。由于诱导效应,氯取代基可称为吸电子基团。
The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is additive; more chlorine atoms have an overall stronger effect, which explains the increasing acidity from mono, to di-, to tri-chlorinated acetic acid. The following diagram shows the inductive effect of trichloro acetate as an example.
感应效应是电负性原子通过σ键产生的电荷分散效应。感应效应是相加的;氯原子越多,总体效果越强,这解释了从一氯乙酸到二氯乙酸,再到三氯乙酸的酸度不断增加的原因。下图以三氯乙酸盐的诱导效果为例。
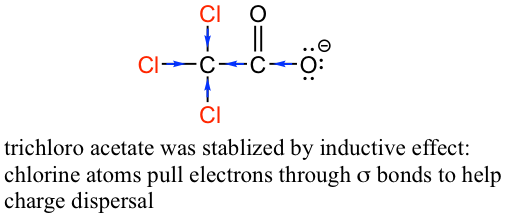
Figure 3.4c Trichloro acetate was stabilized by inductive effect
图3.4c 三氯乙酸通过诱导效应稳定
Because the inductive effect depends on EN, fluorine substituents have a stronger inductive effect than chlorine substituents, making trifluoroacetic acid (TFA) a very strong organic acid.
由于诱导效应取决于EN,氟取代基比氯取代基具有更强的诱导效应,使得三氟乙酸(TFA)成为很强的有机酸。
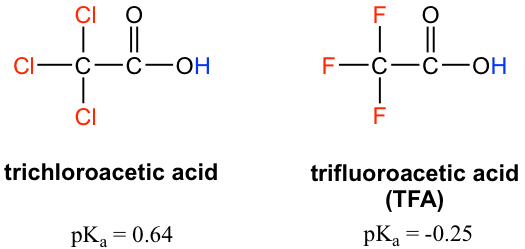
Figure 3.4d trichloroacetic acid (pKa = 0.64) and trifluoroacetic acid (TFA) (pKa = -0.25)
图3.4d 三氯乙酸(pKa = 0.64)和三氟乙酸(TFA)(pKa = -0.25)
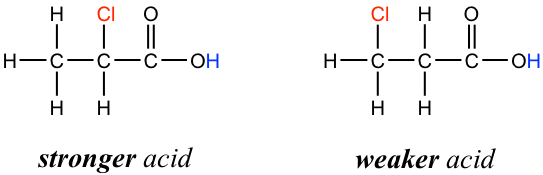
In addition, because the inductive effect takes place through covalent bonds, its influence decreases significantly with distance — thus a chlorine that is two carbons away from a carboxylic acid group has a weaker effect compared to a chlorine just one carbon away.
此外,由于诱导效应是通过共价键发生的,因此其影响力随着距离的增加而显着减弱,因此与羧酸基团相距两个碳的氯的效果比仅相距一个碳的氯的效果更弱。
3.4.4 Hybridization Effect
3.4.4 杂交效应
To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne.
为了介绍杂化效应,我们先看一下烷烃、烯烃和炔烃之间的酸度差异。
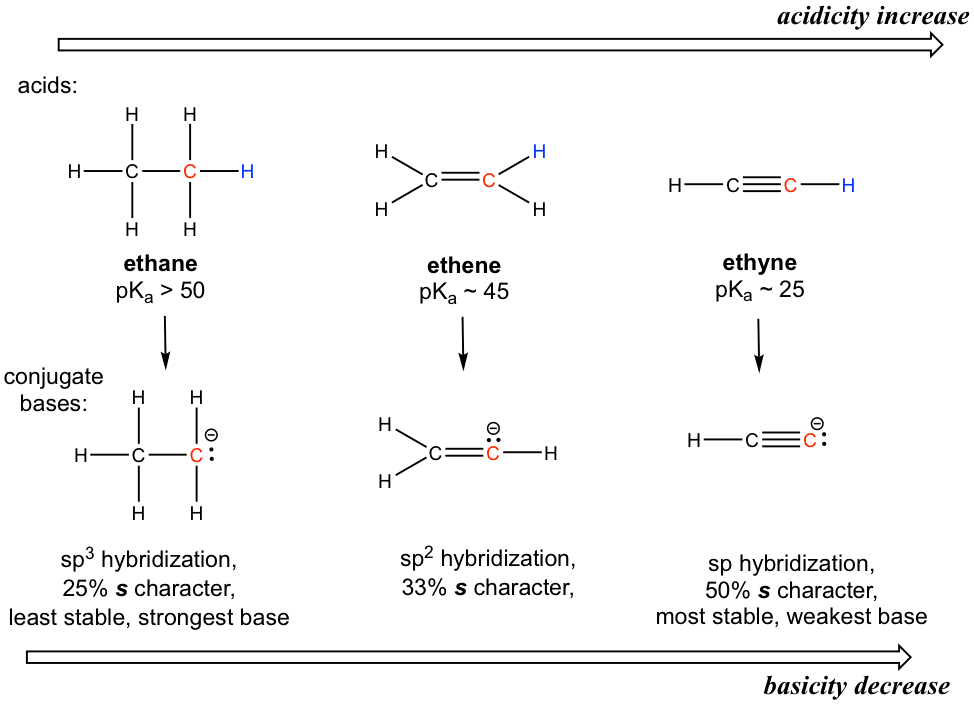
The hydrogen atom is bonded with a carbon atom in all three functional groups, so the element effect does not occur. Also, considering the conjugate base of each, there is no possible extra resonance contributor.
所有三个官能团中的氢原子均与碳原子键合,因此不会发生元素效应。此外,考虑到每个碱基的共轭,不可能有额外的共振贡献者。
The key difference between the conjugate base anions is the hybridization of the carbon atom, which is sp3, sp2 and sp for alkane, alkene and alkyne, respectively. Different hybridizations lead to different s character, which is the percent of s orbitals out of the total number of orbitals. The sp3 hybridization means 25% s character (one s and three p orbitals, so s character is 1/4 = 25%), sp2 hybridization has 33.3% s character, and the number is 50% for sp hybridization. Electrons of 2s orbitals are in a lower energy level than those of 2p orbitals because 2s is much closer to the nucleus. So, for an anion with more s character, the electrons are closer to the nucleus and experience stronger attraction; therefore, the anion has lower energy and is more stable.
共轭碱阴离子之间的主要区别在于碳原子的杂化,烷烃、烯烃和炔烃的碳原子杂化分别为 sp 3 、 sp 2 和 sp 。不同的杂交导致不同的特征,即山梨醇占轨道总数的百分比。 sp 3 杂交意味着 25% s 特征(一个 s 和三个 p 轨道,因此 s 特征为 1/4 = 25%),sp 2 杂交具有 33.3% s 特征, sp杂交的比例为50%。 2s 轨道的电子比 2p 轨道的电子处于更低的能级,因为 2s 更靠近原子核。因此,对于具有更多 s 特性的阴离子,电子离原子核更近,受到的吸引力更强;因此,阴离子的能量较低且更稳定。
The relative stability of the three anions (conjugate bases) can also be illustrated by the electrostatic potential map, in which the lighter color (less red) indicates less electron density of the anion and higher stability.
三种阴离子(共轭碱基)的相对稳定性也可以通过静电势图来说明,其中颜色越浅(红色越少)表明阴离子的电子密度越小,稳定性越高。
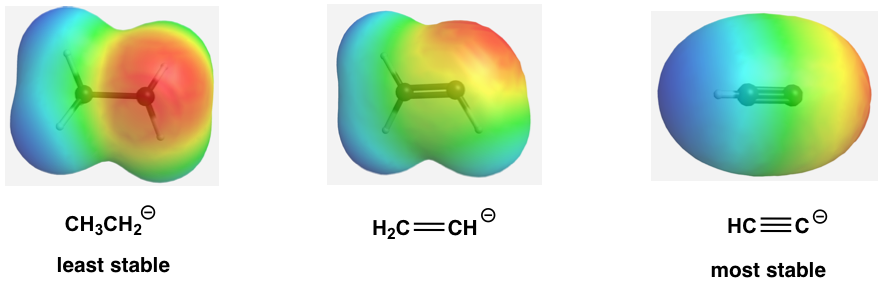
Figure 3.4e Electrostatic potential map of the conj. bases
图 3.4e 连接器的静电势图。基地
This can also be stated in a more general way as more s character in the hybrid orbitals makes the atom more electronegative. For the same atom, an sp hybridized atom is more electronegative than an sp2 hybridized atom, which is more electronegative than an sp3 hybridized atom.
这也可以用更一般的方式来表述,因为杂化轨道中更多的 s 特征使原子更具电负性。对于同一原子,sp 杂化原子比 sp 2 杂化原子更具电负性,而 sp 3 杂化原子比 sp 3 杂化原子更具电负性。