3.2 Organic Acids and Bases and Organic Reaction Mechanism
3.2 жңүжңәй…ёгҖҒжңүжңәзўұеҸҠжңүжңәеҸҚеә”жңәзҗҶ
3.2.1 Organic Acids 3.2.1 жңүжңәй…ё
The acids we discussed in general chemistry usually refer to inorganic acids, such as HCl, H2SO4, and HF. If the structure of the acid contains a вҖңcarbonвҖқ part, then it is an organic acid. Organic acids donate protons in the same way as inorganic acids, but their structure may be more complicated due to the nature of organic structures.
жҲ‘们еңЁжҷ®йҖҡеҢ–еӯҰдёӯи®Ёи®әзҡ„й…ёйҖҡеёёжҳҜжҢҮж— жңәй…ёпјҢдҫӢеҰӮHClгҖҒH 2 SO 4 е’ҢHFгҖӮеҰӮжһңй…ёзҡ„з»“жһ„еҗ«жңүвҖңзўівҖқйғЁеҲҶпјҢеҲҷе®ғжҳҜжңүжңәй…ёгҖӮжңүжңәй…ёд»ҘдёҺж— жңәй…ёзӣёеҗҢзҡ„ж–№ејҸжҸҗдҫӣиҙЁеӯҗпјҢдҪҶз”ұдәҺжңүжңәз»“жһ„зҡ„жҖ§иҙЁпјҢе®ғ们зҡ„з»“жһ„еҸҜиғҪжӣҙеӨҚжқӮгҖӮ
Carboxylic acid, with the general formula of R-COOH, is the most common organic acid we are familiar with. Acetic acid (CH3COOH), an ingredient in vinegar, is a simple example of a carboxylic acid. The Ka of acetic acid is 1.8Г—10-5.
зҫ§й…ёпјҢйҖҡејҸдёәR-COOHпјҢжҳҜжҲ‘们жңҖзҶҹжӮүзҡ„жңүжңәй…ёгҖӮйҶӢй…ё (CH 3 COOH) жҳҜйҶӢдёӯзҡ„дёҖз§ҚжҲҗеҲҶпјҢжҳҜзҫ§й…ёзҡ„дёҖдёӘз®ҖеҚ•дҫӢеӯҗгҖӮд№ҷй…ёзҡ„K a дёә1.8Г—10 -5 гҖӮ
Another common organic acid is the organic derivative of sulfuric acid H2SO4.
еҸҰдёҖз§Қеёёи§Ғзҡ„жңүжңәй…ёжҳҜзЎ«й…ёзҡ„жңүжңәиЎҚз”ҹзү©H 2 SO 4 гҖӮ
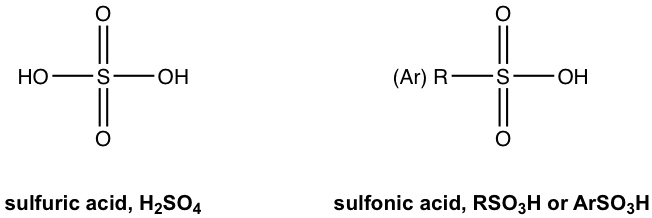
The replacement of one OH group in H2SO4 with a carbon-containing R (alkyl) or Ar (aromatic) group leads to the organic acid named вҖңsulfonic acidвҖқ with the general formula of RSO3H, or ArSO3H. Sulfonic acid is a strong organic acid with a Ka in the range of 106. The structure of a specific sulfonic acid example called p-toluenesulfonic acid is shown here:
з”Ёеҗ«зўізҡ„ RпјҲзғ·еҹәпјүжҲ– ArпјҲиҠійҰҷж—ҸпјүеҹәеӣўеҸ–д»Ј H 2 SO 4 дёӯзҡ„дёҖдёӘ OH еҹәеӣўпјҢеҫ—еҲ°з§°дёәвҖңзЈәй…ёвҖқзҡ„жңүжңәй…ёпјҢе…¶з»“жһ„ејҸдёәпјҡйҖҡејҸдёәRSO 3 HпјҢжҲ–ArSO 3 HгҖӮзЈәй…ёжҳҜдёҖз§Қејәжңүжңәй…ёпјҢK a еңЁ10 6
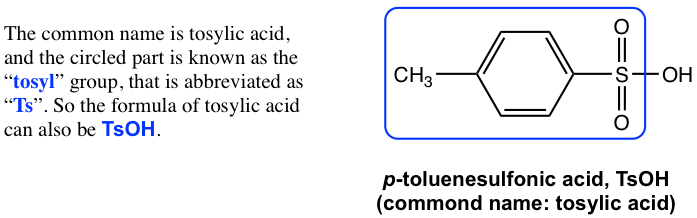
Figure 3.1a CH3C6H4SO3H Tosylic acid
еӣҫ 3.1a CH3C6H4SO3H з”ІиӢҜзЈәй…ё
Other than the acids mentioned here, technically, any organic compound could be an acid because organic compounds always have hydrogen atoms that could potentially be donated as H+. A few examples are shown here with the hydrogen atoms highlighted in blue:
д»ҺжҠҖжңҜдёҠи®ІпјҢйҷӨдәҶиҝҷйҮҢжҸҗеҲ°зҡ„й…ёд№ӢеӨ–пјҢд»»дҪ•жңүжңәеҢ–еҗҲзү©йғҪеҸҜд»ҘжҳҜй…ёпјҢеӣ дёәжңүжңәеҢ–еҗҲзү©жҖ»жҳҜеҗ«жңүж°ўеҺҹеӯҗпјҢжңүеҸҜиғҪд»Ҙ H+ зҡ„еҪўејҸжҸҗдҫӣгҖӮиҝҷйҮҢжҳҫзӨәдәҶдёҖдәӣзӨәдҫӢпјҢе…¶дёӯж°ўеҺҹеӯҗд»Ҙи“қиүІзӘҒеҮәжҳҫзӨәпјҡ

Therefore, the scope of acids has been extended to be broadly extended in an organic chemistry context. We will have further discussions on the acidity of organic compounds in section 3.3, and we will see more acid-base reactions applied to organic compounds later in this chapter.
еӣ жӯӨпјҢй…ёзҡ„иҢғеӣҙе·Іжү©еұ•еҲ°жңүжңәеҢ–еӯҰйўҶеҹҹгҖӮжҲ‘们е°ҶеңЁ 3.3 иҠӮдёӯиҝӣдёҖжӯҘи®Ёи®әжңүжңәеҢ–еҗҲзү©зҡ„й…ёжҖ§пјҢ并且еңЁжң¬з« еҗҺйқўжҲ‘们е°ҶзңӢеҲ°жӣҙеӨҡеә”з”ЁдәҺжңүжңәеҢ–еҗҲзү©зҡ„й…ёзўұеҸҚеә”гҖӮ
3.2.2 Organic BasesВ 3.2.2 жңүжңәзўұ
иҷҪ然з”ұдәҺжҖ»жҳҜж¶үеҸҠж°ўеҺҹеӯҗпјҢиҜҶеҲ«жңүжңәй…ёзӣёеҜ№з®ҖеҚ•пјҢдҪҶиҜҶеҲ«жңүжңәзўұ并дёҚжҖ»жҳҜе®№жҳ“гҖӮж №жҚ®е®ҡд№үпјҢзўұеҹәжҳҜиғҪеӨҹжҺҘеҸ—иҙЁеӯҗзҡ„зү©иҙЁгҖӮжңүжңәзўұеҸҜиғҪж¶үеҸҠеҗ„з§ҚдёҚеҗҢзҡ„з»“жһ„пјҢдҪҶе®ғ们еҝ…йЎ»е…·жңүе…ұеҗҢзҡ„зү№еҫҒпјҢеҚіе…·жңүиғҪеӨҹжҺҘеҸ—иҙЁеӯҗзҡ„з”өеӯҗеҜ№гҖӮз”өеӯҗеҜ№еҸҜд»ҘжҳҜдёӯжҖ§жҲ–еёҰиҙҹз”өзү©иҙЁдёҠзҡ„еӯӨеҜ№з”өеӯҗжҲ–ПҖз”өеӯҗеҜ№гҖӮеӣ жӯӨпјҢжңүжңәзўұеҸҜиғҪж¶үеҸҠд»ҘдёӢзұ»еһӢпјҡ
Negatively charged organic bases: ROвҖ“(alkyloxide), RNHвҖ“(amide), and RвҖ“(alkide, the conjugate base of alkane). Since the negatively charged bases have a high electron density, they are usually stronger bases than the neutral ones.
еёҰиҙҹз”өиҚ·зҡ„жңүжңәзўұпјҡRO-пјҲзғ·еҹәж°§еҢ–зү©пјүгҖҒRNH-пјҲй…°иғәпјүе’ҢR-пјҲйҶҮзӣҗпјҢзғ·зғғзҡ„е…ұиҪӯзўұпјүгҖӮз”ұдәҺеёҰиҙҹз”өзҡ„зўұеҹәе…·жңүй«ҳз”өеӯҗеҜҶеәҰпјҢеӣ жӯӨе®ғ们йҖҡеёёжҜ”дёӯжҖ§зўұеҹәжӣҙејәгҖӮ
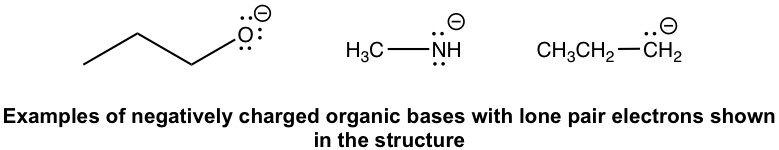
Note: Keep in mind that lone pairs are usually omittedin organic structures as mentioned before. For example, with the formula of CH3NHвҖ“ given, you should understand that the N actually has two pairs of lone pair electrons (as shown in the above structure) and it is a base.
жіЁж„ҸпјҡиҜ·и®°дҪҸпјҢеҰӮеүҚжүҖиҝ°пјҢжңүжңәз»“жһ„дёӯйҖҡеёёдјҡзңҒз•ҘеӯӨеҜ№з”өеӯҗеҜ№гҖӮдҫӢеҰӮпјҢз»ҷеҮәCH 3 NH вҖ“ зҡ„е…¬ејҸпјҢдҪ еә”иҜҘжҳҺзҷҪNе®һйҷ…дёҠжңүдёӨеҜ№еӯӨеҜ№з”өеӯҗпјҲеҰӮдёҠйқўзҡ„з»“жһ„жүҖзӨәпјүпјҢе®ғжҳҜдёҖдёӘеҹәең°гҖӮ
Neutral organic bases, for example amine, C=O group and C=C group
дёӯжҖ§жңүжңәзўұпјҢдҫӢеҰӮиғәгҖҒC=Oеҹәеӣўе’ҢC=Cеҹәеӣў
Amine: RNH2, R2NH, R3N, ArNH2, etc. (section 2.3). As organic derivatives of NH3, which is an inorganic weak base, amines are organic weak bases with lone pair electrons on N that are able to accept the protons.
иғәпјҡRNH 2 гҖҒR 2 NHгҖҒR 3 NгҖҒArNH 2, зӯүпјҲ2.3иҠӮпјүгҖӮдҪңдёәж— жңәејұзўұNH 3 зҡ„жңүжңәиЎҚз”ҹзү©пјҢиғәжҳҜNдёҠе…·жңүиғҪеӨҹжҺҘеҸ—иҙЁеӯҗзҡ„еӯӨеҜ№з”өеӯҗзҡ„жңүжңәејұзўұгҖӮ
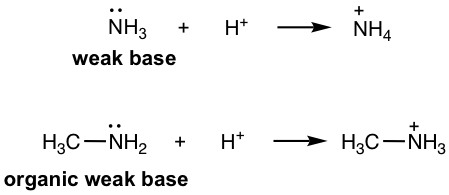
Figure 3.1b weak base and organic weak base
еӣҫ3.1b ејұзўұе’Ңжңүжңәејұзўұ
Functional groups containing oxygen atoms: carbonyl group C=O, alcohol R-OH, and ether R-O-R. The lone pair electrons on O in these groups are able to accept the protons, so functional groups like aldehyde, ketone, alcohol and ether are all organic bases. It may not be easy to accept this concept at first because these groups do not really look like bases. However, they are bases according to the definition because they are able to accept the proton with the lone pair on the oxygen atom.
еҗ«ж°§еҺҹеӯҗзҡ„е®ҳиғҪеӣўпјҡзҫ°еҹәC=OгҖҒйҶҮR-OHгҖҒйҶҡR-O-RгҖӮиҝҷдәӣеҹәеӣўдёӯOдёҠзҡ„еӯӨеҜ№з”өеӯҗиғҪеӨҹжҺҘеҸ—иҙЁеӯҗпјҢеӣ жӯӨйҶӣгҖҒй…®гҖҒйҶҮгҖҒйҶҡзӯүе®ҳиғҪеӣўйғҪжҳҜжңүжңәзўұгҖӮдёҖејҖе§ӢжҺҘеҸ—иҝҷдёӘжҰӮеҝөеҸҜиғҪ并дёҚе®№жҳ“пјҢеӣ дёәиҝҷдәӣеӣўдҪ“зңӢиө·жқҘ并дёҚеғҸеҹәең°гҖӮ然иҖҢпјҢж №жҚ®е®ҡд№үпјҢе®ғ们жҳҜзўұпјҢеӣ дёәе®ғ们иғҪеӨҹжҺҘеҸ—ж°§еҺҹеӯҗдёҠе…·жңүеӯӨеҜ№з”өеӯҗзҡ„иҙЁеӯҗгҖӮ
Adjust your thinking here to embrace thebroader scopeof acids and bases in an organic chemistry context.
еңЁжӯӨи°ғж•ҙжӮЁзҡ„жҖқз»ҙпјҢд»Ҙж¶өзӣ–жңүжңәеҢ–еӯҰиғҢжҷҜдёӢжӣҙе№ҝжіӣзҡ„й…ёе’ҢзўұиҢғеӣҙгҖӮ
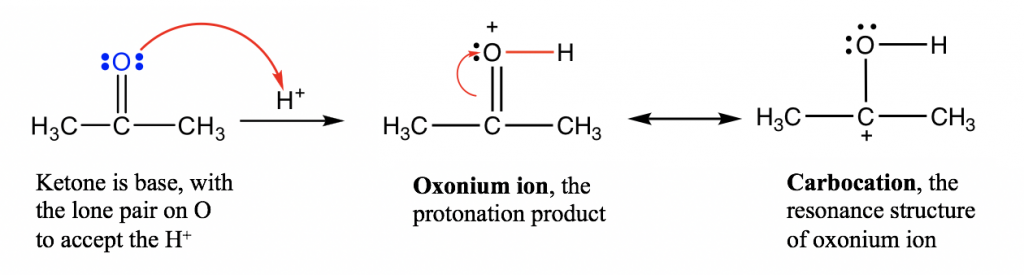
Here, we will take the reaction between acetone and H+ as an example, to understand the reaction deeply by exploring the reaction mechanism,and learn how to use the curved arrows to show it.
еңЁиҝҷйҮҢпјҢжҲ‘们д»Ҙдёҷй…®дёҺH + зҡ„еҸҚеә”дёәдҫӢпјҢйҖҡиҝҮжҺўзҙўеҸҚеә”жңәзҗҶжқҘж·ұе…ҘзҗҶи§ЈиҜҘеҸҚеә”пјҢ并еӯҰд№ еҰӮдҪ•дҪҝз”ЁејҜжӣІз®ӯеӨҙжқҘиЎЁзӨәгҖӮ
A reaction mechanismis the step-by-step electron transfer process that converts reactants to products. Curved arrowsare used to illustrate the reaction mechanism. Curved arrows should always start at the electrons, and end in the spot that is receiving the electrons. The curved arrows used here are similar to those for resonance structures (section 1.4) but are not exactly the same though. Please note that in resonance structures, curved arrows are used to show how the electrons are transferred withinthe molecule, leading to another resonance structure. For mechanism purposes, there must be arrows that connect between species.
еҸҚеә”жңәзҗҶжҳҜе°ҶеҸҚеә”зү©иҪ¬еҢ–дёәдә§зү©зҡ„йҖҗжӯҘз”өеӯҗиҪ¬з§»иҝҮзЁӢгҖӮејҜжӣІз®ӯеӨҙз”ЁдәҺиҜҙжҳҺеҸҚеә”жңәзҗҶгҖӮејҜжӣІз®ӯеӨҙеә”е§Ӣз»Ҳд»Һз”өеӯҗејҖе§ӢпјҢ并еңЁжҺҘ收з”өеӯҗзҡ„зӮ№з»“жқҹгҖӮиҝҷйҮҢдҪҝз”Ёзҡ„ејҜжӣІз®ӯеӨҙдёҺе…ұжҢҜз»“жһ„пјҲ第 1.4 иҠӮпјүзҡ„ејҜжӣІз®ӯеӨҙзӣёдјјпјҢдҪҶ并дёҚе®Ңе…ЁзӣёеҗҢгҖӮиҜ·жіЁж„ҸпјҢеңЁе…ұжҢҜз»“жһ„дёӯпјҢејҜжӣІз®ӯеӨҙз”ЁдәҺжҳҫзӨәз”өеӯҗеҰӮдҪ•еңЁеҲҶеӯҗеҶ…иҪ¬з§»пјҢд»ҺиҖҢеҪўжҲҗеҸҰдёҖдёӘе…ұжҢҜз»“жһ„гҖӮеҮәдәҺжңәеҲ¶зӣ®зҡ„пјҢзү©з§Қд№Ӣй—ҙеҝ…йЎ»жңүиҝһжҺҘзҡ„з®ӯеӨҙгҖӮ
Notes for the above mechanism:
дёҠиҝ°жңәеҲ¶зҡ„жіЁж„ҸдәӢйЎ№пјҡ
For the acid-base reaction between the C=O group and the proton, the arrow starts from the electron pair on O and points to the H+ that is receiving the electron pair. A new O-H bond is formed as a result of this electron pair movement.
еҜ№дәҺ C=O еҹәеӣўе’ҢиҙЁеӯҗд№Ӣй—ҙзҡ„й…ёзўұеҸҚеә”пјҢз®ӯеӨҙд»Һ O дёҠзҡ„з”өеӯҗеҜ№ејҖе§ӢпјҢжҢҮеҗ‘жҺҘ收з”өеӯҗеҜ№зҡ„ H + гҖӮз”ұдәҺз”өеӯҗеҜ№иҝҗеҠЁпјҢеҪўжҲҗдәҶж–°зҡ„ O-H й”®гҖӮIn this acid-base reaction, ketone is protonated by H+ so this reaction can also be called the вҖңprotonation of ketoneвҖқ.
еңЁиҝҷдёӘй…ёзўұеҸҚеә”дёӯпјҢй…®иў«H + иҙЁеӯҗеҢ–пјҢжүҖд»ҘиҝҷдёӘеҸҚеә”д№ҹеҸҜд»Ҙз§°дёәвҖңй…®зҡ„иҙЁеӯҗеҢ–вҖқгҖӮThe product of the protonation is called an вҖңoxonium ionвҖқ, which is stabilized with another resonance structure, carbocation.
иҙЁеӯҗеҢ–зҡ„дә§зү©з§°дёәвҖңж°§йҺ“зҰ»еӯҗвҖқпјҢе®ғйҖҡиҝҮеҸҰдёҖз§Қе…ұжҢҜз»“жһ„зўіжӯЈзҰ»еӯҗжқҘзЁіе®ҡгҖӮ
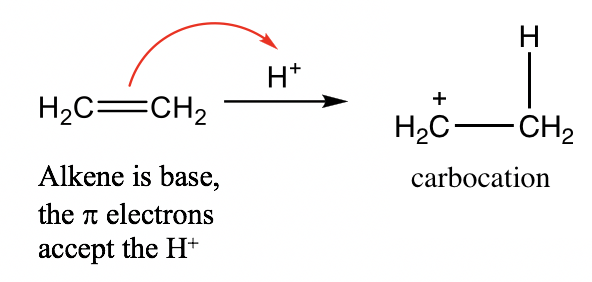
Alkene (C=C): Although there are no lone pair electrons in the C=C bond of alkene, the ПҖ electrons of the C=C double bond are able to accept protons and acts as a base. For example:
зғҜзғғпјҲC=CпјүпјҡиҷҪ然зғҜзғғзҡ„C=Cй”®дёӯжІЎжңүеӯӨеҜ№з”өеӯҗпјҢдҪҶC=CеҸҢй”®зҡ„ПҖз”өеӯҗиғҪеӨҹжҺҘеҸ—иҙЁеӯҗ并充еҪ“зўұеҹәгҖӮдҫӢеҰӮпјҡ
Example: Organic acid and base reaction
зӨәдҫӢпјҡжңүжңәй…ёе’ҢзўұеҸҚеә”
Predict and draw the products of following reaction and use curved arrow to show the mechanism.
йў„жөӢ并画еҮәдёӢеҲ—еҸҚеә”зҡ„дә§зү©пјҢ并用жӣІзәҝз®ӯеӨҙиЎЁзӨәе…¶жңәзҗҶгҖӮ
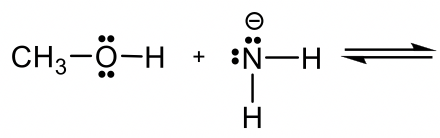
Approach: If H+ is the acid as in previous examples, it is rather easy to predict how the reaction will proceed. However, if there is no obvious acid (or base) as in this example, how do you determine which is the acid, and which is the base?
ж–№жі•пјҡеҰӮжһң H + жҳҜеүҚйқўдҫӢеӯҗдёӯзҡ„й…ёпјҢеҲҷеҫҲе®№жҳ“йў„жөӢеҸҚеә”е°ҶеҰӮдҪ•иҝӣиЎҢгҖӮдҪҶжҳҜпјҢеҰӮжһңеғҸжң¬дҫӢдёҖж ·жІЎжңүжҳҺжҳҫзҡ„й…ёпјҲжҲ–зўұпјүпјҢйӮЈд№ҲеҰӮдҪ•зЎ®е®ҡе“ӘдёӘжҳҜй…ёпјҢе“ӘдёӘжҳҜзўұпјҹ
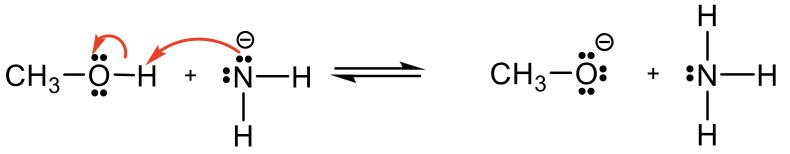
Methanol CH3OH is neutral, and the other reactant, NH2вҖ“, is a negatively charged amide. The amide with a negative charge has higher electron density than the neutral methanol, therefore amide NH2вҖ“should act as base, and CH3OH is the acid that donates H+.
з”ІйҶҮCH 3 OH жҳҜдёӯжҖ§зҡ„пјҢеҸҰдёҖдёӘеҸҚеә”зү©NH 2 вҖ“ жҳҜеёҰиҙҹз”өзҡ„й…°иғәгҖӮеёҰиҙҹз”өиҚ·зҡ„й…°иғәе…·жңүжҜ”дёӯжҖ§з”ІйҶҮжӣҙй«ҳзҡ„з”өеӯҗеҜҶеәҰпјҢеӣ жӯӨй…°иғә NH 2 вҖ“ еә”е……еҪ“зўұпјҢCH 3 OH дёәй…ёжҚҗиө H + гҖӮ
Solution:и§ЈеҶіж–№жЎҲпјҡ

жқҘжәҗдәҺиө„жәҗзҹ©йҳөзҹҘиҜҶжҳҹзҗғгҖҗд»ӘеҷЁеҲҶжһҗеӯҰиӢ‘гҖ‘
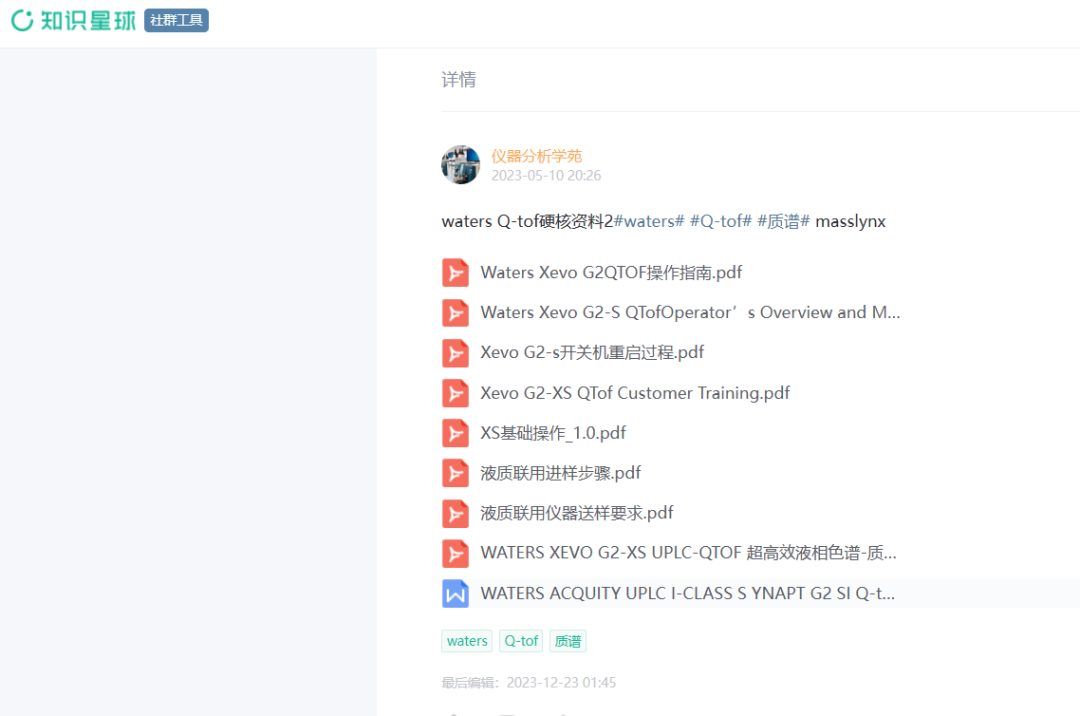
жӣҙеӨҡеҶ…е®№зңӢдёӢиҫ№пјҡ
еҪ“дҪ еңЁд»ӘеҷЁеҲҶжһҗе·ҘдҪңзҡ„ж—¶еҖҷпјҡ
жҳҜеҗҰжӣҫдёәдёҖд»ҪжҠҖжңҜж•ҷзЁӢж— еӨ„жүҫеҲ°иҖҢеҸ‘ж„Ғпјҹ жҳҜеҗҰеңЁйҒҮеҲ°й—®йўҳж—¶еҖҷж— дәәи§Јзӯ”пјҹ жҳҜеҗҰйңҖиҰҒжҸҗеҚҮи®ӨзҹҘзҡ„ж—¶еҖҷgoogleдёҚеҲ°иҮӘе·ұжғіиҰҒзҡ„зӯ”жЎҲпјҹ жҳҜеҗҰйқўеҜ№ж—ҘзӣҠжҝҖзғҲзҡ„еҲҶжһҗиЎҢдёҡж— дәәеҲҶжһҗпјҹ жҳҜеҗҰжғіе‘ҠеҲ«йӮЈдәӣжҲҗеӨ©йғҪжҳҜе№ҝе‘Ҡж»ЎеӨ©йЈһзҡ„иЎҢдёҡзӨҫзҫӨпјҹ иҝҷдәӣй—®йўҳеңЁжҲ‘们зҡ„зҹҘиҜҶжҳҹзҗғйғҪиғҪи§ЈеҶіпјҢиҝҷжҳҜд»ӘеҷЁеҲҶжһҗиЎҢдёҡ(иүІи°ұиҙЁи°ұ)дё“еұһзҡ„зҹҘиҜҶжҳҹзҗғпјҢдёҖдёӘеұһдәҺв–¶д»ӘеҷЁеҲҶжһҗв—ҖиүІи°ұиҙЁи°ұиЎҢдёҡзҡ„дјҳиҙЁд»ҳиҙ№зӨҫзҫӨпјҒ е•Ҷдёҡзҡ„еә•еұӮйҖ»иҫ‘жҳҜд»Җд№ҲпјҢжҳҜжңҖзҹӯж—¶й—ҙеҫ—еҲ°жңҖеҮҶзЎ®зҡ„дҝЎжҒҜпјҢеҠ е…ҘзҹҘиҜҶжҳҹзҗғиғҪеё®дҪ иҠӮзәҰе®қиҙөж—¶й—ҙпјҢи®©дҪ дё“жіЁеӯҰд№ пјҢеҲҶдә«пјҢдәӨжөҒпјҒи®©дҪ дё“жіЁдәҺеӯҰд№ гҖҒеҲҶдә«гҖҒдәӨжөҒе’Ңе•ҶдёҡжҙҪи°ҲгҖӮйҖҡиҝҮжңҖдҪҺзҡ„жҲҗжң¬пјҢдҪ еҸҜд»ҘиҺ·еҫ—жңҖеӨ§зҡ„д»·еҖјгҖӮ
з»ҸиҝҮиҝ‘200дёӘж—Ҙж—ҘеӨңеӨңзҡ„з§ҜзҙҜпјҢд»ӘеҷЁеҲҶжһҗеӯҰиӢ‘зҹҘиҜҶжҳҹзҗғе·Із»ҸеӣҠжӢ¬дәҶи¶…иҝҮ100+д»Ҫдә§е“ҒжҠҖжңҜж–ҮжЎЈпјҢи§Ҷйў‘пјҢз»ҸйӘҢеҲҶдә«пјҢиЎҢдёҡжҠҘе‘ҠзӯүзӯүгҖӮ
иҝҳиғҪеҠ е…Ҙзү№е®ҡдәӨжөҒзҫӨеҫ—еҲ°жң¬дәәе’Ңеҗ„дҪҚеӨ§дҪ¬зҡ„жҢҮеҜј
еҸҠе…¶дё°еҜҢзҡ„д№ҰзұҚиө„жәҗпјҢд№ҹеҸҜд»ҘжҸҗдҫӣзӣёе…ізҡ„жҠҖжңҜе’ЁиҜў

йҡҸзқҖиө„ж–ҷйҖҗжёҗеўһеҠ пјҢдјҡе‘ҳдәәж•°йҖҗжёҗеўһеӨҡпјҢжҜҸйҡ”еҚҠе№ҙпјҢжҳҹзҗғд»·ж јдјҡдҫқжҚ®жғ…еҶөзӣёеә”дёҠжө®пјҢж—©еҠ е…ҘпјҢж—©дә«еҸ—пјҢзӯүеҫ…е°ұжҳҜжҲҗжң¬пјҒ
жҳҹзҗғд»·ж јпјҢдёҚиҝҮдёҖйЎҝйҘӯпјҢдҪҶжҳҜжҳҹзҗғеҚҙеҸҜд»ҘжҢҒз»ӯзҡ„з»ҷдҪ жҸҗдҫӣд»·еҖјпјҢеҠ©еҠӣеёӮеңәејҖжӢ“пјҢй”Җе”®еўһй•ҝпјҢиҒҢеңәжҸҗеҚҮгҖӮиҝҳиғҪжү©еӨ§иЎҢдёҡй“ҫжҺҘпјҢеўһеҠ еҲӣеҜҢеҸҜиғҪпјҒ
зӣ®еүҚжү«з Ғе°ұеҸҜд»ҘеҠ е…Ҙпјҡ
зҹҘиҜҶжҳҹзҗғ1пјҡд»ӘеҷЁеҲҶжһҗеӯҰ家зҡ„еҹ№е…»еҹәең°пјҡд»ӘеҷЁеҲҶжһҗеӯҰиӢ‘

зҹҘиҜҶжҳҹзҗғ2пјҡеҸӘйңҖиҰҒжҹҗжҹҗе…ғпјҢдёҖйЎҝиҢ¶ж°ҙй’ұпјҢжү«з ҒжңүжғҠе–ңпјҢеҢ»иҚҜжҠ•иө„гҖҒдәҶи§ЈеҢ»иҚҜеҲӣдёҡпјҢжҠ•иө„пјҢиҒҢдёҡзҡ„е№іеҸ°пјҢйҷ„еёҰзҫӨиө„жәҗ

vxпјҡеҠ жҲ‘еҫ®дҝЎе’ЁиҜўпјҡе…іжіЁе…¬дј—еҸ·-жҲ‘зҡ„-иҒ”зі»жҲ‘们пјҢжү«з ҒеҠ
еҢ»иҚҜд»ӘеҷЁеҲӣдёҡж–№йқўзҡ„зҫӨиҒҠпјҡйӮҖиҜ·еҲ¶жҲ–иҖ…еҠ е…ҘзҹҘиҜҶжҳҹзҗғ В В В В В В В В В жҖ»з»“дёҚжҳ“пјҢеӨ§дҪ¬иӮҜиөһиөҸеҗҰпјҹ END
еЈ°жҳҺпјҡжң¬е…¬дј—еҸ·жүҖжңүиҪ¬иҪҪж–Үз« зі»еҮәдәҺдј йҖ’жӣҙеӨҡдҝЎжҒҜд№Ӣзӣ®зҡ„пјҢдё”жҳҺзЎ®жіЁжҳҺжқҘжәҗе’ҢдҪңиҖ…пјҢдёҚеёҢжңӣиў«иҪ¬иҪҪзҡ„еӘ’дҪ“жҲ–дёӘдәәеҸҜдёҺжҲ‘们иҒ”зі»пјҢжҲ‘们е°Ҷз«ӢеҚіиҝӣиЎҢеҲ йҷӨеӨ„зҗҶгҖӮжүҖжңүж–Үз« д»…д»ЈиЎЁдҪңиҖ…и§ӮзӮ№пјҢдёҚд»ЈиЎЁжң¬з«ҷз«ӢеңәгҖӮ
ж–№жі•ејҖеҸ‘В В В иҪҜ件ж•ҷзЁӢ
еһӮзӣҙж–Үз«
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-й…ёзўұе’ҢKaзҡ„еӣһйЎҫ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-е®ҳиғҪеӣўзҡ„жңүжңәеҢ–еҗҲзү©зҡ„е‘ҪеҗҚ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-2.2 зғ·зғғзҡ„е‘ҪеҗҚ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-2.3 е®ҳиғҪеӣў
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-2.1 зғҜзғғзҡ„з»“жһ„
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-1.6 д»·й”®зҗҶи®әдёҺжқӮеҢ–
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-1.5 д»·еЈіз”өеӯҗеҜ№жҺ’ж–ҘзҗҶи®әпјҲVSEPRпјү


