4.1 Conformation Analysis of Alkanes
4.1 зғ·зғғзҡ„жһ„иұЎеҲҶжһҗ
4.1.1 Conformation 4.1.1 жһ„иұЎ
At a molecular level, a property of Пғ (sigma) bonds in alkane is that the bonds keep on rotating. For the example of ethane (CH3CH3), one methyl (CH3) group is able to rotate around the C-C bond freely without any obstacles.
еңЁеҲҶеӯҗж°ҙе№ідёҠпјҢзғ·зғғдёӯ Пғ (sigma) й”®зҡ„дёҖдёӘзү№жҖ§жҳҜй”®дёҚж–ӯж—ӢиҪ¬гҖӮд»Ҙд№ҷзғ·пјҲCH 3 CH 3 пјүдёәдҫӢпјҢдёҖдёӘз”ІеҹәпјҲCH 3 пјүеҸҜд»Ҙеӣҙз»•C-Cй”®иҮӘз”ұж—ӢиҪ¬пјҢжІЎжңүд»»дҪ•йҡңзўҚгҖӮ
It is highly recommended that the molecular model is used here to вҖңseeвҖқ the bond rotation. With a molecular model on hand, you can hold one methyl group steady, and rotate the other methyl group.
ејәзғҲе»әи®®еңЁиҝҷйҮҢдҪҝз”ЁеҲҶеӯҗжЁЎеһӢжқҘвҖңжҹҘзңӢвҖқй”®ж—ӢиҪ¬гҖӮжңүдәҶзҺ°жңүзҡ„еҲҶеӯҗжЁЎеһӢпјҢжӮЁеҸҜд»ҘзЁіе®ҡең°дҝқжҢҒдёҖдёӘз”ІеҹәпјҢ并ж—ӢиҪ¬еҸҰдёҖдёӘз”ІеҹәгҖӮ
The C-C bond is formed by the sp3-sp3 orbitals overlapping and the bond is cylindrically symmetrical, so rotation about the bond can occur easily and the molecule does not seem to change. However, a closer look indicates that the rotation of the C-C bond does result in a different spatial arrangement of hydrogen atoms in the molecule, as shown below:
C-Cй”®жҳҜз”ұsp 3 -sp 3 иҪЁйҒ“йҮҚеҸ еҪўжҲҗзҡ„пјҢиҜҘй”®жҳҜеңҶжҹұеҜ№з§°зҡ„пјҢеӣ жӯӨеҫҲе®№жҳ“еҸ‘з”ҹз»•й”®зҡ„ж—ӢиҪ¬пјҢ并且еҲҶеӯҗдјјд№ҺжІЎжңүеҸҳеҢ–гҖӮ然иҖҢпјҢд»”з»Ҷи§ӮеҜҹеҸ‘зҺ°пјҢC-Cй”®зҡ„ж—ӢиҪ¬зЎ®е®һеҜјиҮҙдәҶеҲҶеӯҗдёӯж°ўеҺҹеӯҗзҡ„дёҚеҗҢз©әй—ҙжҺ’еҲ—пјҢеҰӮдёӢжүҖзӨәпјҡ
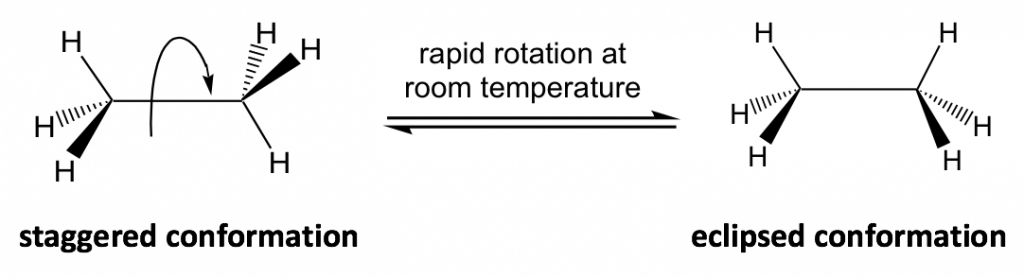
Figure 4.1a Two conformers of ethane in perspective formulas
еӣҫ 4.1a йҖҸи§Ҷе…¬ејҸдёӯд№ҷзғ·зҡ„дёӨдёӘжһ„иұЎејӮжһ„дҪ“
The different spatial arrangements of the atoms/groups that result from the single bond rotation are called conformations. Molecules with different conformations are called conformational isomers or conformers. The two extreme conformations of ethane coming from the C-C rotation shown above are: the staggered conformation with all of the H atoms spread out and the eclipsed conformation with all of the H atoms overlapped.
з”ұеҚ•й”®ж—ӢиҪ¬дә§з”ҹзҡ„еҺҹеӯҗ/еҹәеӣўзҡ„дёҚеҗҢз©әй—ҙжҺ’еҲ—з§°дёәжһ„иұЎгҖӮе…·жңүдёҚеҗҢжһ„иұЎзҡ„еҲҶеӯҗз§°дёәжһ„иұЎејӮжһ„дҪ“жҲ–жһ„иұЎејӮжһ„дҪ“гҖӮдёҠйқўжҳҫзӨәзҡ„жқҘиҮӘ C-C ж—ӢиҪ¬зҡ„д№ҷзғ·зҡ„дёӨз§ҚжһҒз«Ҝжһ„иұЎжҳҜпјҡжүҖжңү H еҺҹеӯҗеұ•ејҖзҡ„дәӨй”ҷжһ„иұЎе’ҢжүҖжңү H еҺҹеӯҗйҮҚеҸ зҡ„йҮҚеҸ жһ„иұЎгҖӮ
In the study of conformation, it is convenient to use certain types of structural formulas. The formula used in the drawing above is the perspective formula (see section 2.1.1) that shows the side-view of the molecule. In perspective formulas, solid and dashed wedges are used to show the spatial arrangement of atoms (or groups) around the sp3 carbons.
еңЁжһ„иұЎз ”究дёӯпјҢдҪҝз”Ёжҹҗдәӣзұ»еһӢзҡ„з»“жһ„ејҸжҳҜеҫҲж–№дҫҝзҡ„гҖӮдёҠеӣҫдёӯдҪҝз”Ёзҡ„е…¬ејҸжҳҜжҳҫзӨәеҲҶеӯҗдҫ§и§Ҷеӣҫзҡ„йҖҸи§Ҷе…¬ејҸпјҲеҸӮи§Ғ第2.1.1иҠӮпјүгҖӮеңЁйҖҸи§Ҷе…¬ејҸдёӯпјҢе®һзәҝе’ҢиҷҡзәҝжҘ”еҪўз”ЁдәҺжҳҫзӨә sp 3 зўіе‘ЁеӣҙеҺҹеӯҗпјҲжҲ–еҹәеӣўпјүзҡ„з©әй—ҙжҺ’еҲ—гҖӮ
Another structural formula is the sawhorse formula which shows the tilted top-view of the molecule.
еҸҰдёҖдёӘз»“жһ„ејҸжҳҜй”ҜжңЁжһ¶ејҸпјҢе®ғжҳҫзӨәдәҶеҲҶеӯҗзҡ„еҖҫж–ңйЎ¶и§ҶеӣҫгҖӮ
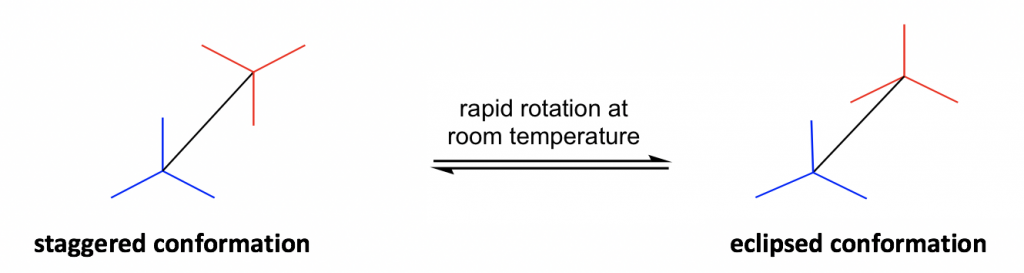
Figure 4.1b Two conformers of ethane in sawhorse formulas
еӣҫ 4.1b й”ҜжңЁжһ¶е…¬ејҸдёӯд№ҷзғ·зҡ„дёӨз§Қжһ„иұЎејӮжһ„дҪ“
The most commonly applied formula in conformation analysis is the Newman projection formula.
жһ„иұЎеҲҶжһҗдёӯжңҖеёёз”Ёзҡ„е…¬ејҸжҳҜзәҪжӣјжҠ•еҪұе…¬ејҸгҖӮ
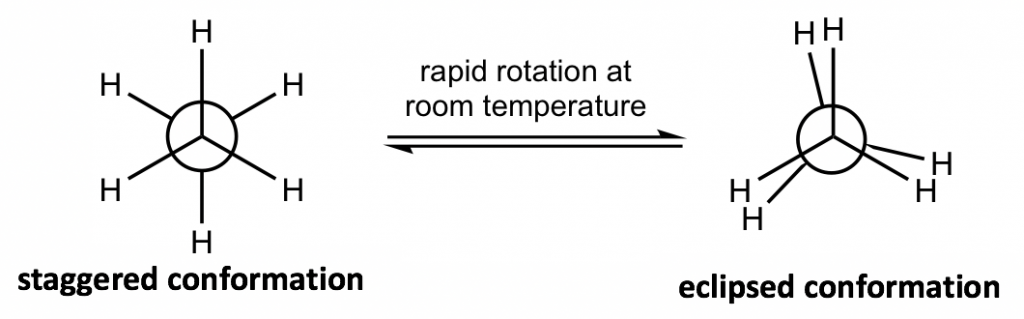
Figure 4.1c Two conformers of ethane in Newman projections
еӣҫ 4.1c зәҪжӣјжҠ•еҪұдёӯд№ҷзғ·зҡ„дёӨдёӘжһ„иұЎејӮжһ„дҪ“
How to draw a Newman projection
еҰӮдҪ•з»ҳеҲ¶зәҪжӣјжҠ•еҪұ
To draw a Newman projection, we will imagine viewing the molecule from one carbon to the next carbon atom directly along a selected CвҖ“C bond, as shown below, and follow the rules:
дёәдәҶз»ҳеҲ¶зәҪжӣјжҠ•еҪұпјҢжҲ‘们е°ҶжғіиұЎзӣҙжҺҘжІҝзқҖйҖүе®ҡзҡ„ C-C й”®д»ҺдёҖдёӘзўіеҺҹеӯҗеҲ°дёӢдёҖдёӘзўіеҺҹеӯҗзҡ„еҲҶеӯҗпјҢеҰӮдёӢжүҖзӨәпјҢ并йҒөеҫӘ规еҲҷпјҡ
Figure 4.1d Viewing of the molecule
еӣҫ 4.1d еҲҶеӯҗи§Ҷеӣҫ
The front carbon atom is shown as a point with three other bonds:
еүҚйқўзҡ„зўіеҺҹеӯҗжҳҫзӨәдёәеёҰжңүе…¶д»–дёүдёӘй”®зҡ„зӮ№пјҡ
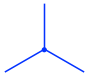
The rear carbon atom is shown as a circle with three other bonds:
еҗҺйқўзҡ„зўіеҺҹеӯҗжҳҫзӨәдёәеёҰжңүе…¶д»–дёүдёӘй”®зҡ„еңҶеңҲпјҡ
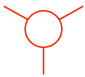
Put the two carbons together to get the Newman projection of the staggered conformation:
е°ҶдёӨдёӘзўіж”ҫеңЁдёҖиө·еҚіеҸҜеҫ—еҲ°дәӨй”ҷжһ„иұЎзҡ„зәҪжӣјжҠ•еҪұпјҡ
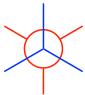
From the staggered conformation, fix the front carbon in place and rotate the rear carbon by 60В° to get the eclipsed conformation:
д»ҺдәӨй”ҷжһ„иұЎдёӯпјҢе°ҶеүҚзўіеӣәе®ҡеҲ°дҪҚпјҢ并е°ҶеҗҺзўіж—ӢиҪ¬ 60В°пјҢеҫ—еҲ°йҮҚеҸ жһ„иұЎпјҡ
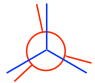
Note: In eclipsed conformers, the C-H bonds are supposed to be completely overlapped; however, to make the rear groups still visible, the bonds on the rear carbon are intentionally drawn slightly tilted.
жіЁж„ҸпјҡеңЁйҮҚеҸ жһ„иұЎејӮжһ„дҪ“дёӯпјҢC-H й”®еә”иҜҘе®Ңе…ЁйҮҚеҸ пјӣ然иҖҢпјҢдёәдәҶдҪҝеҗҺеҹәеӣўд»Қ然еҸҜи§ҒпјҢеҗҺзўідёҠзҡ„й”®ж•…ж„ҸзЁҚеҫ®еҖҫж–ңгҖӮ
4.1.2 Conformation Analysis of Ethane
4.1.2 д№ҷзғ·зҡ„жһ„иұЎеҲҶжһҗ
Next, we will do a conformation analysis of ethane by using the Newman projections. A conformation analysis is an investigation of the energy differences and relative stabilities of the different conformations of a compound.
жҺҘдёӢжқҘпјҢжҲ‘们е°ҶдҪҝз”ЁзәҪжӣјжҠ•еҪұеҜ№д№ҷзғ·иҝӣиЎҢжһ„иұЎеҲҶжһҗгҖӮжһ„иұЎеҲҶжһҗжҳҜеҜ№еҢ–еҗҲзү©дёҚеҗҢжһ„иұЎзҡ„иғҪйҮҸе·®ејӮе’ҢзӣёеҜ№зЁіе®ҡжҖ§зҡ„з ”з©¶гҖӮ
The two conformations of ethane, staggered and eclipsed, are different and therefore should be in different energy levels. You may also intuitively predict that the staggered conformation is more stable and is lower energy because the C-H bonds are arranged as far apart as possible in that conformation. That is correct! In eclipsed conformations, the H atoms on the front carbon are overlapping with the H atoms on the rear carbon, and this arrangement causes the repulsion between the electrons of C-H bonds of the two carbons. This type of repulsion is called torsional strain, also known as eclipsing strain. Due to torsional strain, the eclipsed conformer is in an energy level that is 12 kJ/mol (or about 2.9 kcal/mol) higher than the staggered one. This can be represented graphically in a potential energy diagram as shown in Figure 4.1f.
д№ҷзғ·зҡ„дёӨз§Қжһ„иұЎпјҢдәӨй”ҷжһ„иұЎе’ҢйҮҚеҸ жһ„иұЎжҳҜдёҚеҗҢзҡ„пјҢеӣ жӯӨеә”иҜҘеӨ„дәҺдёҚеҗҢзҡ„иғҪзә§гҖӮжӮЁиҝҳеҸҜд»Ҙзӣҙи§Ӯең°йў„жөӢдәӨй”ҷжһ„иұЎжӣҙзЁіе®ҡ并且иғҪйҮҸжӣҙдҪҺпјҢеӣ дёәеңЁиҜҘжһ„иұЎдёӯ C-H й”®жҺ’еҲ—еҫ—е°ҪеҸҜиғҪиҝңгҖӮйӮЈжҳҜеҜ№зҡ„пјҒеңЁйҮҚеҸ жһ„иұЎдёӯпјҢеүҚзўідёҠзҡ„HеҺҹеӯҗдёҺеҗҺзўідёҠзҡ„HеҺҹеӯҗйҮҚеҸ пјҢиҝҷз§ҚжҺ’еҲ—еҜјиҮҙдёӨдёӘзўізҡ„C-Hй”®з”өеӯҗд№Ӣй—ҙзҡ„жҺ’ж–ҘгҖӮиҝҷз§Қзұ»еһӢзҡ„жҺ’ж–Ҙз§°дёәжүӯиҪ¬еә”еҸҳпјҢд№ҹз§°дёәйЈҹеә”еҸҳгҖӮз”ұдәҺжүӯиҪ¬еә”еҸҳпјҢйҮҚеҸ жһ„иұЎејӮжһ„дҪ“зҡ„иғҪзә§жҜ”дәӨй”ҷжһ„иұЎејӮжһ„дҪ“й«ҳ 12 kJ/molпјҲжҲ–зәҰ 2.9 kcal/molпјүгҖӮиҝҷеҸҜд»Ҙз”ЁеҠҝиғҪеӣҫжқҘиЎЁзӨәпјҢеҰӮеӣҫ 4.1f жүҖзӨәгҖӮ
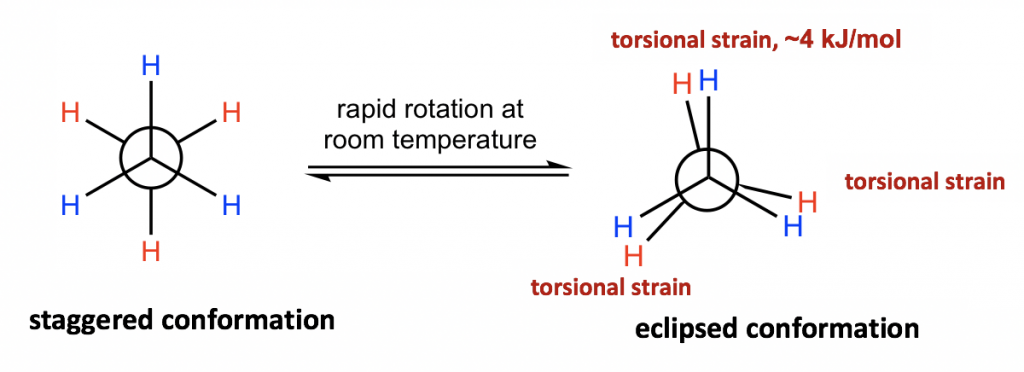
Figure 4.1e Staggered vs. eclipsed conformation
еӣҫ 4.1e дәӨй”ҷжһ„иұЎдёҺйҮҚеҸ жһ„иұЎ
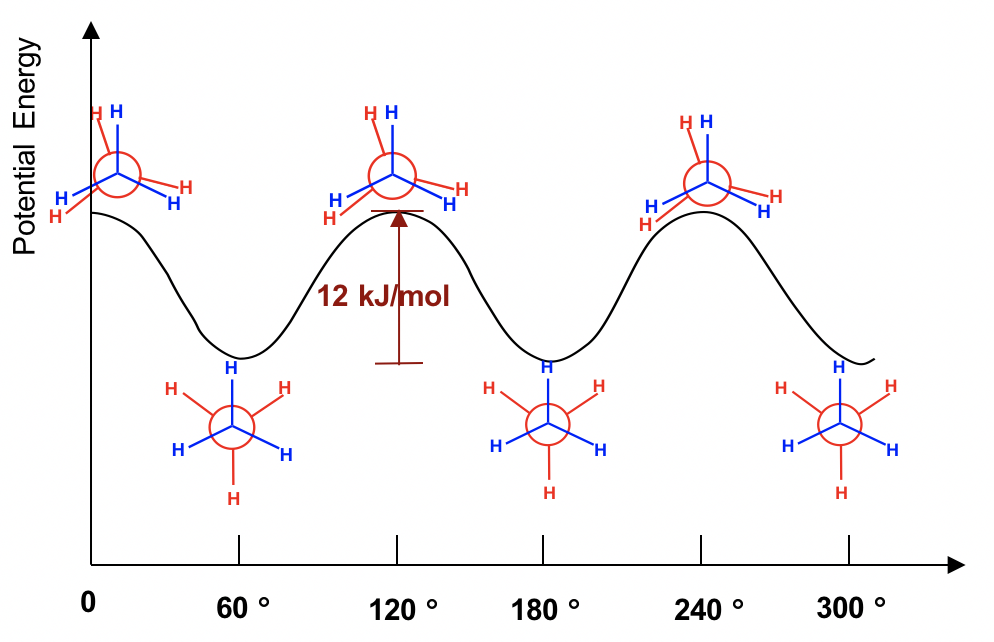
Fig. 4.1f Potential Energy В of Ethane vs the Angle of Rotation about the C-C bond
еӣҫ 4.1f д№ҷзғ·еҠҝиғҪдёҺ C-C й”®ж—ӢиҪ¬и§’еәҰзҡ„е…ізі»
Because of this energy difference, an energy barrier must be overcome when rotation about the C-C bond occurs. However, this energy difference in ethane is small, and the kinetic energy of molecules at room temperature is high enough to cover it. So, at room temperature, the changes from staggered to eclipsed conformers occur millions of times per second. Because of these continuous interconversions, these two conformers cannot be separated from each other. However, at any given moment, about 99% of the ethane molecules will be in a staggered conformation because of their higher stability.
з”ұдәҺиҝҷз§ҚиғҪйҮҸе·®ејӮпјҢеҪ“еҸ‘з”ҹеӣҙз»• C-C й”®зҡ„ж—ӢиҪ¬ж—¶пјҢеҝ…йЎ»е…ӢжңҚиғҪйҮҸеҠҝеһ’гҖӮ然иҖҢпјҢд№ҷзғ·дёӯзҡ„иҝҷз§ҚиғҪйҮҸе·®еҫҲе°ҸпјҢ并且е®Өжё©дёӢеҲҶеӯҗзҡ„еҠЁиғҪи¶іеӨҹй«ҳжқҘиҰҶзӣ–е®ғгҖӮеӣ жӯӨпјҢеңЁе®Өжё©дёӢпјҢд»ҺдәӨй”ҷжһ„иұЎејӮжһ„дҪ“еҲ°йҮҚеҸ жһ„иұЎејӮжһ„дҪ“зҡ„еҸҳеҢ–жҜҸз§’еҸ‘з”ҹж•°зҷҫдёҮж¬ЎгҖӮз”ұдәҺиҝҷдәӣиҝһз»ӯзҡ„зӣёдә’иҪ¬еҢ–пјҢиҝҷдёӨдёӘжһ„иұЎејӮжһ„дҪ“дёҚиғҪеҪјжӯӨеҲҶзҰ»гҖӮ然иҖҢпјҢеңЁд»»дҪ•з»ҷе®ҡж—¶еҲ»пјҢеӨ§зәҰ 99% зҡ„д№ҷзғ·еҲҶеӯҗе°ҶеӨ„дәҺдәӨй”ҷжһ„иұЎпјҢеӣ дёәе®ғ们具жңүжӣҙй«ҳзҡ„зЁіе®ҡжҖ§гҖӮ
4.1.3 Conformation Analysis of Propane
4.1.3 дёҷзғ·зҡ„жһ„иұЎеҲҶжһҗ
A similar analysis can be applied to propane as well. There are still two types of conformations: staggered and eclipsed resulting from the rotation. The difference between propane and ethane is that there is a methyl (CH3) group connected on the rear carbon for propane. However, that does not affect the relative stability, and the staggered conformer is more stable and lower energy.
зұ»дјјзҡ„еҲҶжһҗд№ҹйҖӮз”ЁдәҺдёҷзғ·гҖӮд»Қ然жңүдёӨз§Қзұ»еһӢзҡ„жһ„иұЎпјҡдәӨй”ҷжһ„иұЎе’Ңеӣ ж—ӢиҪ¬иҖҢдә§з”ҹзҡ„йҮҚеҸ жһ„иұЎгҖӮдёҷзғ·е’Ңд№ҷзғ·зҡ„еҢәеҲ«еңЁдәҺдёҷзғ·зҡ„еҗҺзўідёҠиҝһжҺҘжңүз”ІеҹәпјҲCH 3 пјүеҹәеӣўгҖӮдҪҶиҝҷ并дёҚеҪұе“ҚзӣёеҜ№зЁіе®ҡжҖ§пјҢдәӨй”ҷжһ„иұЎејӮжһ„дҪ“жӣҙзЁіе®ҡпјҢиғҪйҮҸжӣҙдҪҺгҖӮ
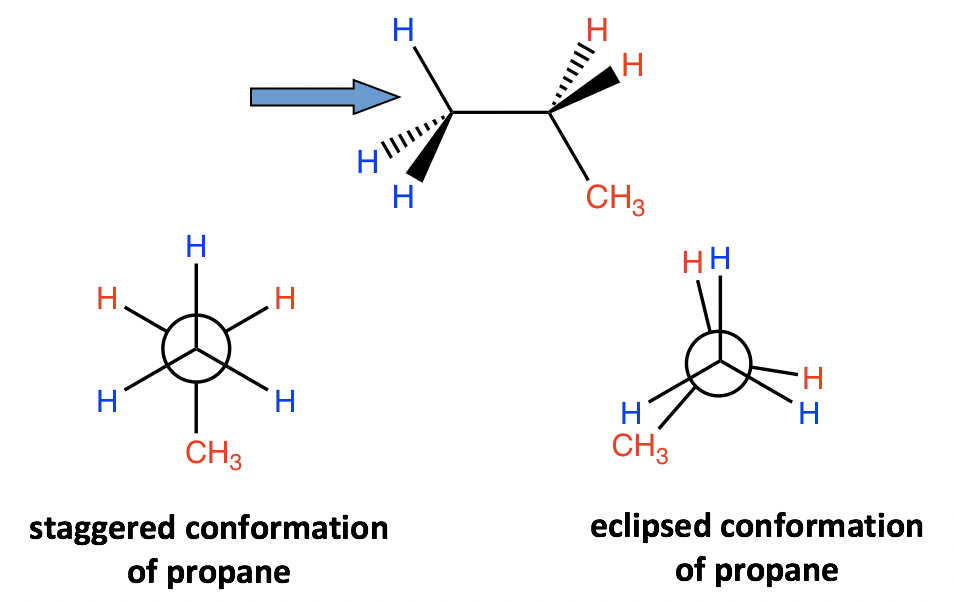
Figure 4.1g Staggered and eclipsed conformation of propane
еӣҫ 4.1g дёҷзғ·зҡ„дәӨй”ҷе’ҢйҮҚеҸ жһ„иұЎ
4.1.4 Conformation Analysis of Butane
4.1.4 дёҒзғ·зҡ„жһ„иұЎеҲҶжһҗ
There are three C-C bonds in butane, and rotation can occur about each of them. If we choose C1-C2 (or C3-C4) for the study, the situation is almost the same as propane, with the ethyl CH2CH3 group replacing the CH3 group. However, if we consider the rotation about the C2-C3 bond, the situation will be much more complex.
дёҒзғ·дёӯжңүдёүдёӘ C-C й”®пјҢжҜҸдёӘй”®йғҪеҸҜд»ҘеҸ‘з”ҹж—ӢиҪ¬гҖӮеҰӮжһңжҲ‘们йҖүжӢ©C1-C2пјҲжҲ–C3-C4пјүиҝӣиЎҢз ”з©¶пјҢжғ…еҶөеҮ д№ҺдёҺдёҷзғ·зӣёеҗҢпјҢз”Ёд№ҷеҹәCH 2 CH 3 еҹәеӣўд»ЈжӣҝCH 3 з»„гҖӮ然иҖҢпјҢеҰӮжһңжҲ‘们иҖғиҷ‘C2-C3й”®зҡ„ж—ӢиҪ¬пјҢжғ…еҶөе°ұдјҡеӨҚжқӮеҫ—еӨҡгҖӮ
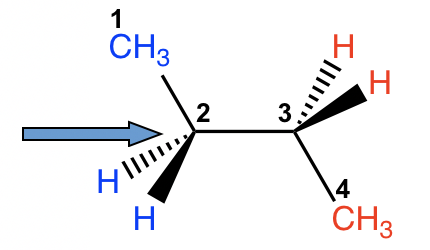
Figure 4.1h Conformation analysis of butane by viewing along C2-C3 bond
еӣҫ4.1h жІҝC2-C3й”®и§ӮеҜҹдёҒзғ·зҡ„жһ„иұЎеҲҶжһҗ
For both carbon atoms, C2 and C3, there are two hydrogen atoms and one methyl CH3 group bonded. We can start with the conformer in which the two CH3 groups are opposite to each other, then fix the front carbon and do 60В° rotations of the rear carbon to investigate all the possible conformations.
еҜ№дәҺдёӨдёӘзўіеҺҹеӯҗпјҲC2 е’Ң C3пјүпјҢжңүдёӨдёӘж°ўеҺҹеӯҗе’ҢдёҖдёӘй”®еҗҲзҡ„з”Іеҹә CH 3 еҹәеӣўгҖӮжҲ‘们еҸҜд»Ҙд»ҺдёӨдёӘCH 3 еҹәеӣўзӣёеҜ№зҡ„жһ„иұЎејҖе§ӢпјҢ然еҗҺеӣәе®ҡеүҚйқўзҡ„зўіпјҢе°ҶеҗҺйқўзҡ„зўіж—ӢиҪ¬60В°жқҘз ”з©¶жүҖжңүеҸҜиғҪзҡ„жһ„иұЎгҖӮ
Exercises 4.1: Draw all the possible conformers of butane from viewing along the C2-C3 bond. Finish this practice by yourself before continue reading!
з»ғд№ 4.1пјҡжІҝзқҖ C2-C3 й”®и§ӮеҜҹпјҢз”»еҮәдёҒзғ·жүҖжңүеҸҜиғҪзҡ„жһ„иұЎејӮжһ„дҪ“гҖӮеңЁз»§з»ӯйҳ…иҜ»д№ӢеүҚпјҢиҜ·иҮӘиЎҢе®ҢжҲҗжӯӨз»ғд№ пјҒ
Tips for drawing all the possible conformers about a certain C-C bond:
з»ҳеҲ¶жҹҗдёӘ C-C й”®зҡ„жүҖжңүеҸҜиғҪжһ„иұЎејӮжһ„дҪ“зҡ„жҠҖе·§пјҡ
View along that C-C bond; circle and decide what atoms/groups are connected on each carbon;
жІҝзқҖ C-C й”®жҹҘзңӢпјӣеңҲеҮә并确е®ҡжҜҸдёӘзўідёҠиҝһжҺҘзҡ„еҺҹеӯҗ/еҹәеӣўпјӣStart with the staggered conformation in which the largest groups on each carbon are opposite (far away) to each other (this is called the вҖңantiвҖқconformation as we will learn later);
д»ҺдәӨй”ҷжһ„иұЎејҖе§ӢпјҢе…¶дёӯжҜҸдёӘзўідёҠжңҖеӨ§зҡ„еҹәеӣўеҪјжӯӨзӣёеҜ№пјҲиҝңпјүпјҲиҝҷз§°дёәвҖңеҸҚвҖқжһ„иұЎпјҢжҲ‘们зЁҚеҗҺе°ҶдәҶи§ЈеҲ°пјүпјӣKeep the groups on one carbon вҖңfixedвҖқ, and rotate the groups on the other carbon at 60В° angles. Repeat the rotation five times, and you should get total of six conformers.
дҝқжҢҒдёҖдёӘзўідёҠзҡ„еҹәеӣўвҖңеӣәе®ҡвҖқпјҢ并д»Ҙ 60В° и§’ж—ӢиҪ¬еҸҰдёҖдёӘзўідёҠзҡ„еҹәеӣўгҖӮйҮҚеӨҚж—ӢиҪ¬дә”ж¬ЎпјҢдҪ еә”иҜҘеҫ—еҲ°жҖ»е…ұе…ӯдёӘжһ„иұЎејӮжһ„дҪ“гҖӮ
Answers to Chapter 4 Practice Questions第 4 з« з»ғд№ йўҳзӯ”жЎҲ
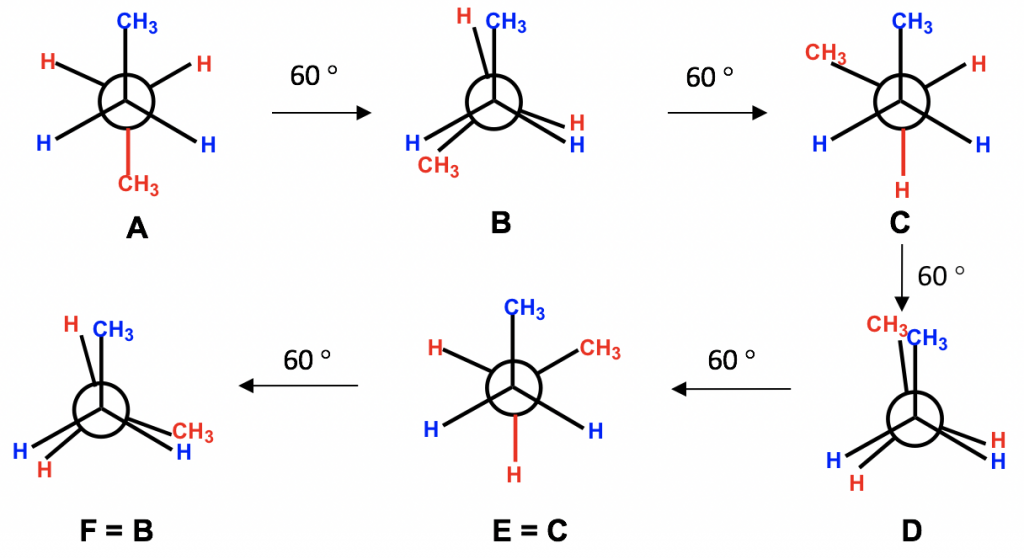
Figure 4.1i All the conformers of butane by viewing along C2-C3 bond
еӣҫ4.1i жІҝC2-C3й”®и§ӮеҜҹдёҒзғ·зҡ„жүҖжңүжһ„иұЎејӮжһ„дҪ“
Among all six conformers obtained, there are three staggered and three eclipsed. Staggered conformations C and E should be in the same energy level because the groups are arranged in an equivalent way between these two conformers. Similarly, eclipsed conformations F and B are also in the same energy level. So, our studies can be focused on the four conformers: A, B, C and D, which are different in terms of energy and stability.
еңЁиҺ·еҫ—зҡ„жүҖжңүе…ӯдёӘжһ„иұЎејӮжһ„дҪ“дёӯпјҢжңүдёүдёӘдәӨй”ҷејӮжһ„дҪ“е’ҢдёүдёӘйҮҚеҸ жһ„иұЎејӮжһ„дҪ“гҖӮдәӨй”ҷжһ„иұЎ C е’Ң E еә”иҜҘеӨ„дәҺзӣёеҗҢзҡ„иғҪзә§пјҢеӣ дёәиҝҷдёӨдёӘжһ„иұЎдҪ“д№Ӣй—ҙзҡ„еҹәеӣўд»Ҙзӯүж•Ҳж–№ејҸжҺ’еҲ—гҖӮеҗҢж ·пјҢйҮҚеҸ жһ„иұЎFе’ҢBд№ҹеӨ„дәҺзӣёеҗҢзҡ„иғҪзә§гҖӮеӣ жӯӨпјҢжҲ‘们зҡ„з ”з©¶еҸҜд»ҘйӣҶдёӯеңЁеӣӣз§Қжһ„иұЎејӮжһ„дҪ“пјҡAгҖҒBгҖҒCе’ҢDпјҢе®ғ们еңЁиғҪйҮҸе’ҢзЁіе®ҡжҖ§ж–№йқўжңүжүҖдёҚеҗҢгҖӮ
Between the two staggered conformers A and C, A is more stable than C because the two methyl CH3 groups in A are as far apart as possible. This most stable staggered conformation is called the antiвҖ“conformation (anti is Greek for вҖңoppositeвҖқ). In antiвҖ“conformations, the largest groups on the front and rear carbon are 180В° opposite to each other. The other staggered conformation C is called a gauche conformation, in which the two large groups are adjacent and are 60В° to each other. With the large groups being close to each other in gauche conformers, the molecule experiences steric strain. Steric strain is the strain that is caused when atoms (or groups) are close enough together that their electron clouds repel each other. Steric strain only matters when the groups are close to each other (less or equal to 60В°), so steric strain does not apply in anti-conformations. The magnitude of steric strain also depends on the size of the group: the larger the size, the higher the steric strain. As a result, there is no steric strain between two small hydrogen atoms, even if they are close to each other.
еңЁдёӨдёӘдәӨй”ҷжһ„иұЎејӮжһ„дҪ“Aе’ҢCд№Ӣй—ҙпјҢAжҜ”CжӣҙзЁіе®ҡпјҢеӣ дёәAдёӯзҡ„дёӨдёӘз”ІеҹәCH3еҹәеӣўи·қзҰ»е°ҪеҸҜиғҪиҝңгҖӮиҝҷз§ҚжңҖзЁіе®ҡзҡ„дәӨй”ҷжһ„иұЎз§°дёәеҸҚжһ„иұЎпјҲanti жҳҜеёҢи…ҠиҜӯпјҢж„ҸдёәвҖңзӣёеҸҚвҖқпјүгҖӮеңЁеҸҚжһ„иұЎдёӯпјҢеүҚеҗҺзўідёҠжңҖеӨ§зҡ„еҹәеӣўеҪјжӯӨжҲҗ180В°зӣёеҜ№гҖӮеҸҰдёҖз§ҚдәӨй”ҷжһ„иұЎCз§°дёәgaucheжһ„иұЎпјҢе…¶дёӯдёӨдёӘеӨ§еҹәеӣўзӣёйӮ»дё”еҪјжӯӨжҲҗ60В°гҖӮз”ұдәҺеӨ§еҹәеӣўеңЁзІ—дҝ—жһ„иұЎдҪ“дёӯеҪјжӯӨйқ иҝ‘пјҢеҲҶеӯҗдјҡз»ҸеҺҶз©әй—ҙеә”еҸҳгҖӮдҪҚйҳ»еә”еҸҳжҳҜеҪ“еҺҹеӯҗпјҲжҲ–еҹәеӣўпјүи¶іеӨҹжҺҘиҝ‘д»ҘиҮідәҺе®ғ们зҡ„з”өеӯҗдә‘зӣёдә’жҺ’ж–Ҙж—¶еј•иө·зҡ„еә”еҸҳгҖӮз©әй—ҙеә”еҸҳд»…еңЁеҹәеӣўеҪјжӯӨжҺҘиҝ‘пјҲе°ҸдәҺжҲ–зӯүдәҺ 60В°пјүж—¶жүҚйҮҚиҰҒпјҢеӣ жӯӨз©әй—ҙеә”еҸҳдёҚйҖӮз”ЁдәҺеҸҚжһ„иұЎгҖӮз©әй—ҙеә”еҸҳзҡ„еӨ§е°ҸиҝҳеҸ–еҶідәҺеҹәеӣўзҡ„еӨ§е°Ҹпјҡе°әеҜёи¶ҠеӨ§пјҢз©әй—ҙеә”еҸҳи¶Ҡй«ҳгҖӮеӣ жӯӨпјҢеҚідҪҝдёӨдёӘе°Ҹж°ўеҺҹеӯҗеҪјжӯӨйқ иҝ‘пјҢе®ғ们д№Ӣй—ҙд№ҹдёҚеӯҳеңЁз©әй—ҙеә”еҸҳгҖӮ
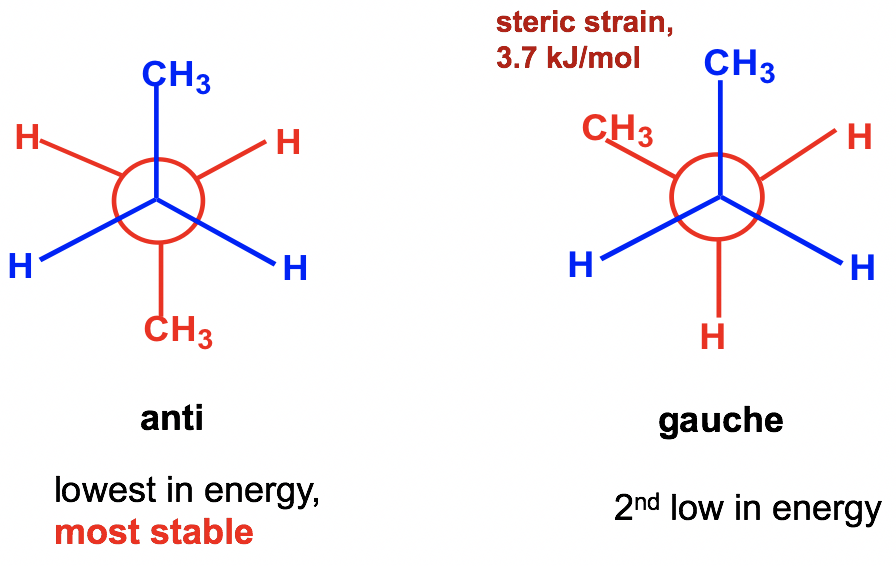
Figure 4.1j Anti and gauche conformations
еӣҫ4.1j Antiе’Ңgaucheжһ„иұЎ
Between the two eclipsed conformers B and D, D is less stable than B because the two CH3 groups are eclipsing (overlapping) each other in D, causing both torsional and steric strains.
еңЁдёӨдёӘйҮҚеҸ жһ„иұЎејӮжһ„дҪ“ B е’Ң D д№Ӣй—ҙпјҢD жҜ” B жӣҙдёҚзЁіе®ҡпјҢеӣ дёәдёӨдёӘ CH 3 еҹәеӣўеңЁ D дёӯеҪјжӯӨйҮҚеҸ пјҲйҮҚеҸ пјүпјҢд»ҺиҖҢеҜјиҮҙжүӯиҪ¬е’Ңз©әй—ҙеә”еҸҳгҖӮ
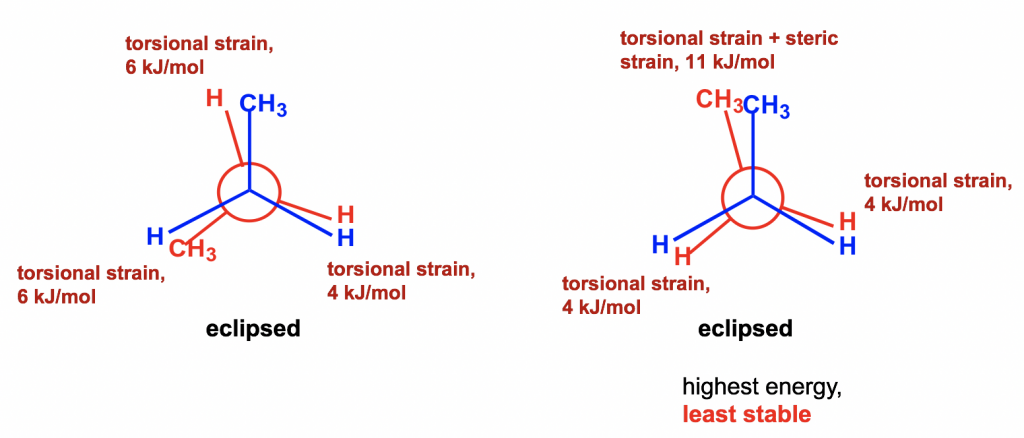
Figure 4.1k Comparison between the two eclipsed conformations
еӣҫ4.1k дёӨз§ҚйҮҚеҸ жһ„иұЎзҡ„жҜ”иҫғ
The energy difference of all the conformers obtained from the rotation about the C2-C3 bond are shown in the potential energy diagram Fig. 4.1l. The curve is more complex than that of ethane since there are four different energy levels corresponding to four conformers with different stabilities. Even the energy barriers for the rotations are larger than that of ethane, but they are still not high enough to stop rotation at room temperature.
з»•C2-C3й”®ж—ӢиҪ¬еҫ—еҲ°зҡ„жүҖжңүжһ„иұЎејӮжһ„дҪ“зҡ„иғҪйҮҸе·®еҰӮеӣҫ4.1lеҠҝиғҪеӣҫжүҖзӨәгҖӮиҜҘжӣІзәҝжҜ”д№ҷзғ·зҡ„жӣІзәҝжӣҙеӨҚжқӮпјҢеӣ дёәжңүеӣӣз§ҚдёҚеҗҢзҡ„иғҪзә§еҜ№еә”дәҺе…·жңүдёҚеҗҢзЁіе®ҡжҖ§зҡ„еӣӣз§Қжһ„иұЎејӮжһ„дҪ“гҖӮеҚідҪҝж—ӢиҪ¬зҡ„иғҪеһ’жҜ”д№ҷзғ·еӨ§пјҢдҪҶе®ғ们д»Қ然дёҚи¶ід»ҘеңЁе®Өжё©дёӢеҒңжӯўж—ӢиҪ¬гҖӮ
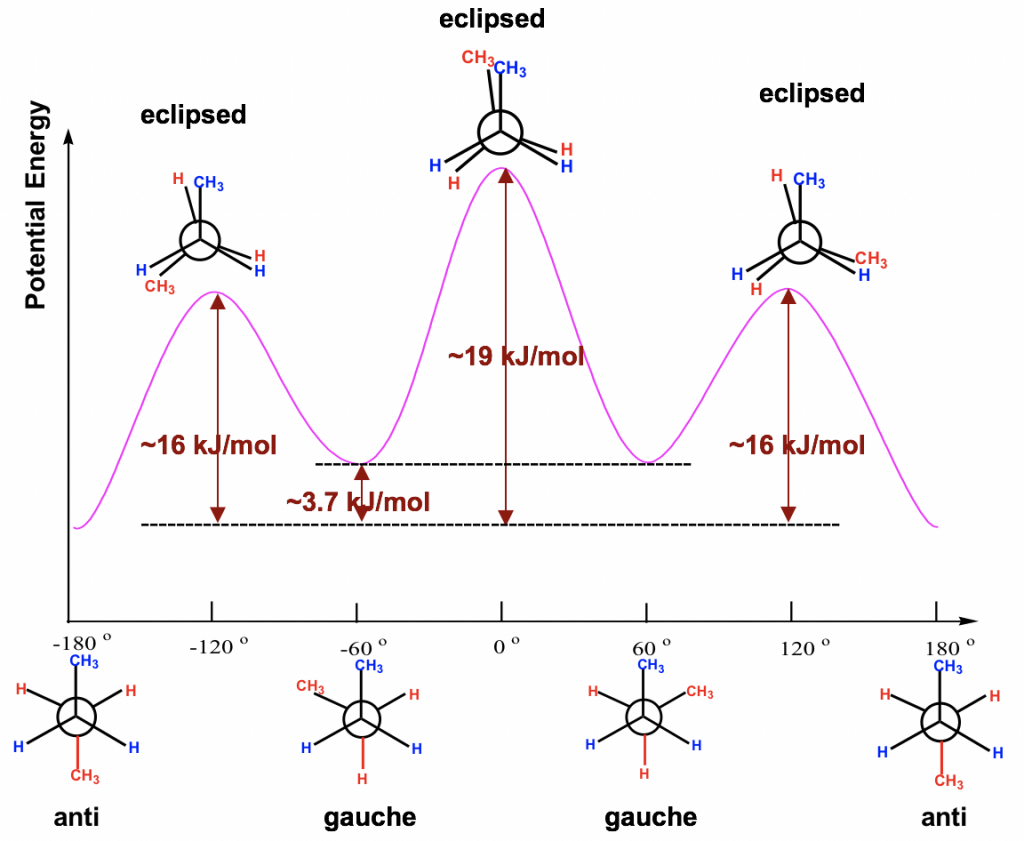
Figure 4.1l Potential Energy of Butane vs the Angle of Rotation about the C2-C3 bond
еӣҫ 4.1l дёҒзғ·еҠҝиғҪдёҺ C2-C3 й”®ж—ӢиҪ¬и§’еәҰзҡ„е…ізі»
жқҘжәҗдәҺиө„жәҗзҹ©йҳөзҹҘиҜҶжҳҹзҗғгҖҗд»ӘеҷЁеҲҶжһҗеӯҰиӢ‘гҖ‘
жӣҙеӨҡеҶ…е®№зңӢдёӢиҫ№пјҡ
еҪ“дҪ еңЁд»ӘеҷЁеҲҶжһҗе·ҘдҪңзҡ„ж—¶еҖҷпјҡ
жҳҜеҗҰжӣҫдёәдёҖд»ҪжҠҖжңҜж•ҷзЁӢж— еӨ„жүҫеҲ°иҖҢеҸ‘ж„Ғпјҹ жҳҜеҗҰеңЁйҒҮеҲ°й—®йўҳж—¶еҖҷж— дәәи§Јзӯ”пјҹ жҳҜеҗҰйңҖиҰҒжҸҗеҚҮи®ӨзҹҘзҡ„ж—¶еҖҷgoogleдёҚеҲ°иҮӘе·ұжғіиҰҒзҡ„зӯ”жЎҲпјҹ жҳҜеҗҰйқўеҜ№ж—ҘзӣҠжҝҖзғҲзҡ„еҲҶжһҗиЎҢдёҡж— дәәеҲҶжһҗпјҹ жҳҜеҗҰжғіе‘ҠеҲ«йӮЈдәӣжҲҗеӨ©йғҪжҳҜе№ҝе‘Ҡж»ЎеӨ©йЈһзҡ„иЎҢдёҡзӨҫзҫӨпјҹ иҝҷдәӣй—®йўҳеңЁжҲ‘们зҡ„зҹҘиҜҶжҳҹзҗғйғҪиғҪи§ЈеҶіпјҢиҝҷжҳҜд»ӘеҷЁеҲҶжһҗиЎҢдёҡ(иүІи°ұиҙЁи°ұ)дё“еұһзҡ„зҹҘиҜҶжҳҹзҗғпјҢдёҖдёӘеұһдәҺв–¶д»ӘеҷЁеҲҶжһҗв—ҖиүІи°ұиҙЁи°ұиЎҢдёҡзҡ„дјҳиҙЁд»ҳиҙ№зӨҫзҫӨпјҒ е•Ҷдёҡзҡ„еә•еұӮйҖ»иҫ‘жҳҜд»Җд№ҲпјҢжҳҜжңҖзҹӯж—¶й—ҙеҫ—еҲ°жңҖеҮҶзЎ®зҡ„дҝЎжҒҜпјҢеҠ е…ҘзҹҘиҜҶжҳҹзҗғиғҪеё®дҪ иҠӮзәҰе®қиҙөж—¶й—ҙпјҢи®©дҪ дё“жіЁеӯҰд№ пјҢеҲҶдә«пјҢдәӨжөҒпјҒи®©дҪ дё“жіЁдәҺеӯҰд№ гҖҒеҲҶдә«гҖҒдәӨжөҒе’Ңе•ҶдёҡжҙҪи°ҲгҖӮйҖҡиҝҮжңҖдҪҺзҡ„жҲҗжң¬пјҢдҪ еҸҜд»ҘиҺ·еҫ—жңҖеӨ§зҡ„д»·еҖјгҖӮ
з»ҸиҝҮиҝ‘200дёӘж—Ҙж—ҘеӨңеӨңзҡ„з§ҜзҙҜпјҢд»ӘеҷЁеҲҶжһҗеӯҰиӢ‘зҹҘиҜҶжҳҹзҗғе·Із»ҸеӣҠжӢ¬дәҶи¶…иҝҮ100+д»Ҫдә§е“ҒжҠҖжңҜж–ҮжЎЈпјҢи§Ҷйў‘пјҢз»ҸйӘҢеҲҶдә«пјҢиЎҢдёҡжҠҘе‘ҠзӯүзӯүгҖӮ
иҝҳиғҪеҠ е…Ҙзү№е®ҡдәӨжөҒзҫӨеҫ—еҲ°жң¬дәәе’Ңеҗ„дҪҚеӨ§дҪ¬зҡ„жҢҮеҜј
еҸҠе…¶дё°еҜҢзҡ„д№ҰзұҚиө„жәҗпјҢд№ҹеҸҜд»ҘжҸҗдҫӣзӣёе…ізҡ„жҠҖжңҜе’ЁиҜў

йҡҸзқҖиө„ж–ҷйҖҗжёҗеўһеҠ пјҢдјҡе‘ҳдәәж•°йҖҗжёҗеўһеӨҡпјҢжҜҸйҡ”еҚҠе№ҙпјҢжҳҹзҗғд»·ж јдјҡдҫқжҚ®жғ…еҶөзӣёеә”дёҠжө®пјҢж—©еҠ е…ҘпјҢж—©дә«еҸ—пјҢзӯүеҫ…е°ұжҳҜжҲҗжң¬пјҒ
жҳҹзҗғд»·ж јпјҢдёҚиҝҮдёҖйЎҝйҘӯпјҢдҪҶжҳҜжҳҹзҗғеҚҙеҸҜд»ҘжҢҒз»ӯзҡ„з»ҷдҪ жҸҗдҫӣд»·еҖјпјҢеҠ©еҠӣеёӮеңәејҖжӢ“пјҢй”Җе”®еўһй•ҝпјҢиҒҢеңәжҸҗеҚҮгҖӮиҝҳиғҪжү©еӨ§иЎҢдёҡй“ҫжҺҘпјҢеўһеҠ еҲӣеҜҢеҸҜиғҪпјҒ
зӣ®еүҚжү«з Ғе°ұеҸҜд»ҘеҠ е…Ҙпјҡ
зҹҘиҜҶжҳҹзҗғ1пјҡд»ӘеҷЁеҲҶжһҗеӯҰ家зҡ„еҹ№е…»еҹәең°пјҡд»ӘеҷЁеҲҶжһҗеӯҰиӢ‘

зҹҘиҜҶжҳҹзҗғ2пјҡеҸӘйңҖиҰҒжҹҗжҹҗе…ғпјҢдёҖйЎҝиҢ¶ж°ҙй’ұпјҢжү«з ҒжңүжғҠе–ңпјҢеҢ»иҚҜжҠ•иө„гҖҒдәҶи§ЈеҢ»иҚҜеҲӣдёҡпјҢжҠ•иө„пјҢиҒҢдёҡзҡ„е№іеҸ°пјҢйҷ„еёҰзҫӨиө„жәҗ

vxпјҡеҠ жҲ‘еҫ®дҝЎе’ЁиҜўпјҡе…іжіЁе…¬дј—еҸ·-жҲ‘зҡ„-иҒ”зі»жҲ‘们пјҢжү«з ҒеҠ
еҢ»иҚҜд»ӘеҷЁеҲӣдёҡж–№йқўзҡ„зҫӨиҒҠпјҡйӮҖиҜ·еҲ¶жҲ–иҖ…еҠ е…ҘзҹҘиҜҶжҳҹзҗғ В В В В В В В В В жҖ»з»“дёҚжҳ“пјҢеӨ§дҪ¬иӮҜиөһиөҸеҗҰпјҹ END
еЈ°жҳҺпјҡжң¬е…¬дј—еҸ·жүҖжңүиҪ¬иҪҪж–Үз« зі»еҮәдәҺдј йҖ’жӣҙеӨҡдҝЎжҒҜд№Ӣзӣ®зҡ„пјҢдё”жҳҺзЎ®жіЁжҳҺжқҘжәҗе’ҢдҪңиҖ…пјҢдёҚеёҢжңӣиў«иҪ¬иҪҪзҡ„еӘ’дҪ“жҲ–дёӘдәәеҸҜдёҺжҲ‘们иҒ”зі»пјҢжҲ‘们е°Ҷз«ӢеҚіиҝӣиЎҢеҲ йҷӨеӨ„зҗҶгҖӮжүҖжңүж–Үз« д»…д»ЈиЎЁдҪңиҖ…и§ӮзӮ№пјҢдёҚд»ЈиЎЁжң¬з«ҷз«ӢеңәгҖӮ
ж–№жі•ејҖеҸ‘В В В иҪҜ件ж•ҷзЁӢ
еһӮзӣҙж–Үз«
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-3.5 и·Ҝжҳ“ж–Ҝй…ёе’Ңи·Ҝжҳ“ж–Ҝзўұ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-е…ғзҙ еҪұе“Қ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-жңүжңәй…ёгҖҒжңүжңәзўұеҸҠжңүжңәеҸҚеә”жңәзҗҶ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-й…ёзўұе’ҢKaзҡ„еӣһйЎҫ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-е®ҳиғҪеӣўзҡ„жңүжңәеҢ–еҗҲзү©зҡ„е‘ҪеҗҚ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-2.2 зғ·зғғзҡ„е‘ҪеҗҚ
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-2.3 е®ҳиғҪеӣў
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-2.1 зғҜзғғзҡ„з»“жһ„
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-1.6 д»·й”®зҗҶи®әдёҺжқӮеҢ–
д»ӘеҷЁеҲҶжһҗеӨ§зҷҫ科全д№Ұ-жңүжңәеҢ–еӯҰ-1.5 д»·еЈіз”өеӯҗеҜ№жҺ’ж–ҘзҗҶи®әпјҲVSEPRпјү


