4.2 Cycloalkanes and Their Relative Stabilities
4.2 环烷烃及其相对稳定性
While the open chain alkanes have conformational isomers because of bond rotation, will this apply to cycloalkanes as well? In this section, we will take a look at properties of cycloalkanes first, then investigate how the different conformers of cycloalkanes contribute to the different stabilities.
虽然开链烷烃由于键旋转而具有构象异构体,但这也适用于环烷烃吗?在本节中,我们将首先了解环烷烃的性质,然后研究环烷烃的不同构象异构体如何影响不同的稳定性。
The short line structural formulas of cycloalkenes simply look like shapes such as a triangle, square, etc. The internal angles of the shapes can be calculated with geometry, as shown below.
环烯烃的短线结构式简单地看起来像三角形、正方形等形状。形状的内角可以用几何来计算,如下所示。
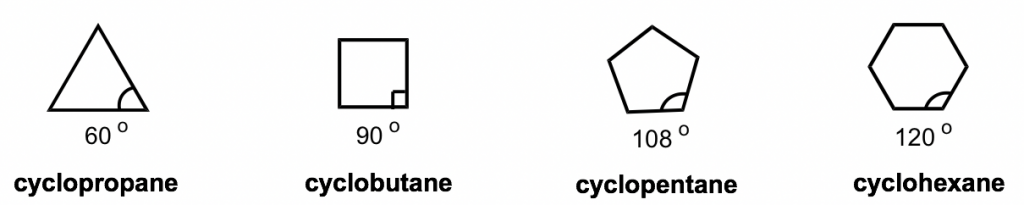
Figure 4.2a Short line structural formula of cycloalkanes
图4.2a 环烷烃的短线结构式
An interesting fact about the cycloalkanes is that they have different relative stabilities, and the stability depends on the size of the ring. It has been observed that cyclic compounds found in nature are usually in 5- or 6-membered rings, and 3- or 4-membered rings are rare.
关于环烷烃的一个有趣的事实是它们具有不同的相对稳定性,并且稳定性取决于环的大小。据观察,自然界中发现的环状化合物通常为5元环或6元环,3元环或4元环很少见。
To explain this stability difference, German chemist Adolf von Baeyer proposed the “Bayer Strain Theory”. By assuming all the rings are in a flat (or planar) shape, Bayer Theory suggests that the difference between the ideal bond angle (which is 109.5° for sp3 carbon) and the angle in the planer cycloalkane causes the strain, which is called angle strain. According to the Bayer Theory, cyclopentane would be the most stable because its bond angle, 108°, is closest to the ideal angle of 109.5°. Cyclopropane would be the least stable one since it has the largest angle deviation of 49.5° (60° vs 109.5°). It was also predicted that cyclohexane would be less stable than cyclopentane because of the larger angle deviation (10.5° deviation for cyclohexane vs 1.5° for cyclopentane), and as the number of sides in the cycloalkanes increases beyond six, the stability decreases.
为了解释这种稳定性差异,德国化学家阿道夫·冯·拜尔提出了“拜耳应变理论”。通过假设所有环均呈扁平(或平面)形状,拜耳理论表明理想键角(sp 3 碳为 109.5°)与平面环烷烃中的角度之间的差异导致应变,称为角应变。根据拜耳理论,环戊烷是最稳定的,因为它的键角为 108°,最接近理想的角度 109.5°。环丙烷是最不稳定的一种,因为它的最大角度偏差为 49.5°(60° 与 109.5°)。还预测环己烷的稳定性比环戊烷差,因为其角度偏差较大(环己烷偏差10.5°,环戊烷偏差1.5°),并且当环烷烃的边数增加到超过6时,稳定性降低。
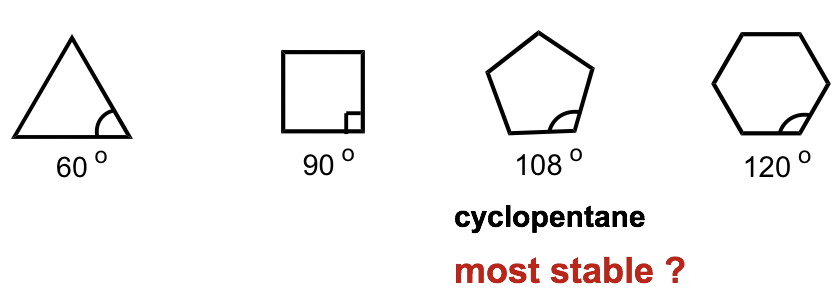
Figure 4.2b Which cyclo is stable?
图 4.2b 哪个环是稳定的?
However, experimental results show a different trend. It turns out that cyclohexane is the most stable ring that is strain-free and is as stable as a chain alkane. Furthermore, cyclic compounds do not become less and less stable as the number of rings increases.
然而,实验结果显示出不同的趋势。事实证明,环己烷是最稳定的环,无应变,与链烷烃一样稳定。此外,环状化合物不会随着环数的增加而变得越来越不稳定。
To measure the relative stability of cycloalkanes, the heat of combustion (ΔHcomb) for each cycloalkane was measured. The heat of combustion is the amount of heat released when the compounds burns completely with oxygen. The cycloalkanes will be in higher energy levels than corresponding chain alkanes because of strain energy. Therefore, when cycloalkane burns, more heat will be released, so the difference of ΔHcomb between cycloalkane vs the “strainless” chain alkane is just the amount of strain energy, as shown below. The larger the difference, the higher the strain energy of the cycloalkane. The strain energy for different cycloalkanes measured by this method are listed in Table 4.1.
为了测量环烷烃的相对稳定性,测量了每种环烷烃的燃烧热(ΔH comb )。燃烧热是化合物与氧气完全燃烧时释放的热量。由于应变能,环烷烃将比相应的链烷烃处于更高的能级。因此,当环烷烃燃烧时,会释放出更多的热量,因此环烷烃与“无应变”链烷烃之间的ΔH comb 之差正是应变能的大小,如下所示。差值越大,环烷烃的应变能越高。用该方法测得的不同环烷烃的应变能列于表4.1。
combustion reaction: (CH2)n + 3n/2 O2 → n CO2 + n H2O + heat
燃烧反应:(CH 2 ) n + 3n/2 O 2 →n CO 2 + n H 2
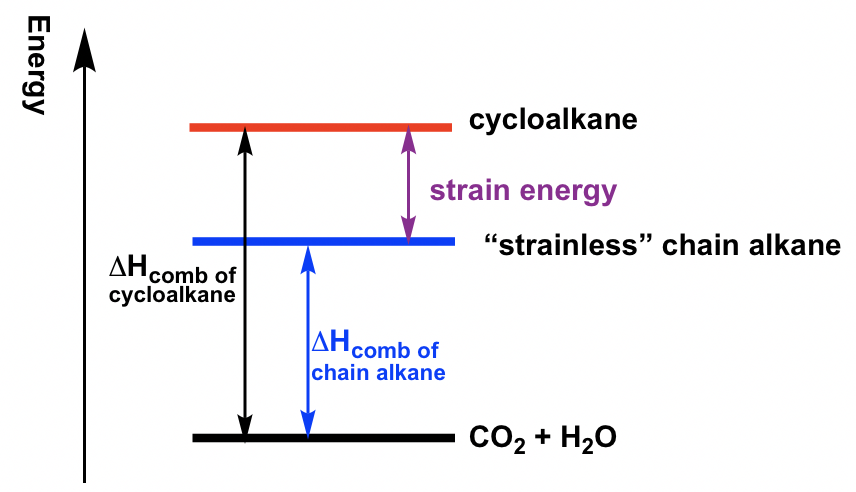
Figure 4.2c The relationship between heat of combustion and strain energy
图4.2c 燃烧热与应变能的关系
cyclopropane 环丙烷 | cyclobutane 环丁烷 | cyclopentane 环戊烷 | cyclohexane 环己烷 | |
Strain Energy (KJ/mol) 应变能 (KJ/mol) | 114 | 110 | 25 | 0 |
Table 4.1 Strain Energies of Cycloalkanes
表 4.1 环烷烃的应变能
The major drawback of the Baeyer Theory is that we must assume that all the rings are flat. The highest stability of cyclohexane from experimental results indicate that the rings may not be in a planar shape. We will have a closer look at the actual shape and conformation of 3-, 4-, 5- and 6-membered cycloalkanes.
拜耳理论的主要缺点是我们必须假设所有环都是平的。实验结果表明环己烷的最高稳定性表明环可能不是平面形状。我们将仔细研究 3、4、5 和 6 元环烷烃的实际形状和构象。
Cyclopropane 环丙烷
With three carbons for the ring, cyclopropane must be planar.
由于环有三个碳,环丙烷必须是平面的。
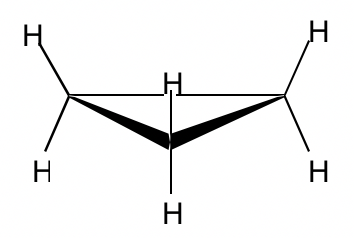
Figure 4.2d Cyclopropane 图 4.2d 环丙烷
The bond angle in cyclopropane is 60°, derived significantly from the optimal angle of 109.5°, so it has very high angle strains. The sp3-sp3 orbitals can only overlap partially because of the angle deviation, so the overlapping is not as effective as it should be, and as a result, the C-C bond in cyclopropane is relatively weak.
环丙烷中的键角为 60°,明显源自最佳角度 109.5°,因此它具有非常高的角应变。由于角度偏差,sp 3 -sp 3 轨道只能部分重叠,因此重叠效果没有应有的效果,因此,中的 C-C 键环丙烷的作用相对较弱。

Because of the poor overlapping of sp3-sp3orbitals, the bonds formed in cyclopropane resemble the shape of a banana and are sometimes called banana bonds.
由于 sp 3 -sp 3 轨道的重叠较差,环丙烷中形成的键类似于香蕉的形状,有时称为香蕉键。
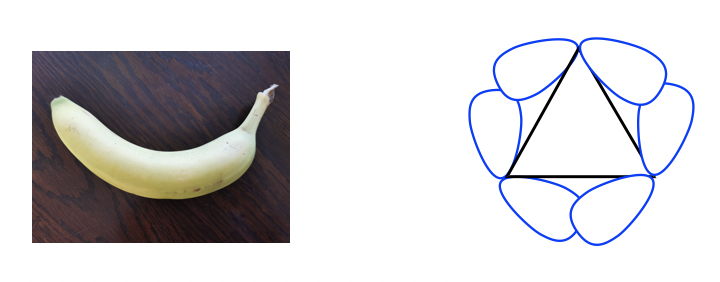
Figure 4.2e “Banana bonds” of cyclopropane
图 4.2e 环丙烷的“香蕉键”
Other than the angle strains, all the adjacent C-H bonds are eclipsed in cyclopropane; therefore, the torsional strains are applied as well. Such strains can be “viewed” more clearly from the Newman projection of cyclopropane.
除角应变外,所有相邻的 C-H 键在环丙烷中都被遮盖;因此,也施加扭转应变。从环丙烷的纽曼投影中可以更清楚地“观察”此类菌株。
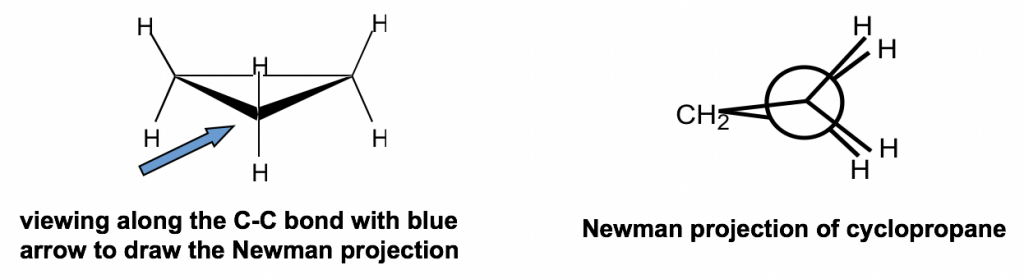
The Newman projection of cyclopropane might seems unusual at first glance. For cyclopropane, there are three carbons, so the CH2 group connects with both front and rear carbons of the Newman projection.
环丙烷的纽曼投影乍一看似乎很不寻常。对于环丙烷,有三个碳,因此 CH 2 基团与纽曼投影的前碳和后碳连接。
Because of the high level of angle strains and torsional strains, 3-membered rings are unstable. They rarely exist in nature and undergo ring-opening reaction easily to release the strains.
由于角应变和扭转应变水平较高,三元环不稳定。它们在自然界中很少存在,并且容易发生开环反应以释放菌株。
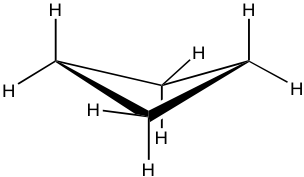
Cyclobutane 环丁烷
Cyclobutane is not planar. The ring puckers (or folds) slightly due to the efforts of releasing some torsional strain. Meanwhile, cyclobutane still has a considerable number of angle strains as the internal angles become about 88° with the folded shape. Overall, cyclobutane is an unstable structure with a high level of strains.
环丁烷不是平面的。由于释放一些扭转应变的努力,环稍微起皱(或折叠)。同时,环丁烷仍然具有相当数量的角应变,因为折叠形状的内角变为约88°。总体而言,环丁烷是一种不稳定的结构,具有高水平的应变。
Cyclopentane 环戊烷
Cyclopentane is also not planar, and the total level of strain is significantly lowered. It also puckers and adopts a bent conformation where one carbon atom sticks out of the plane of the others, which helps to release the torsional strain by allowing some hydrogen atoms to become almost staggered.
环戊烷也不是平面的,总应变水平显着降低。它还会起皱并采用一种弯曲构象,其中一个碳原子伸出其他碳原子的平面,这有助于通过允许一些氢原子几乎交错来释放扭转应变。
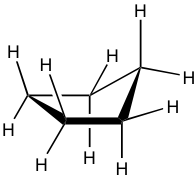
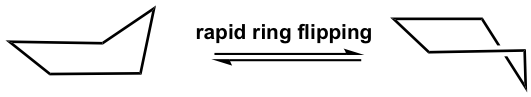
This bent shape of cyclopentane is also called the “envelope” conformation. The envelope conformation can undergo a process called “ring flipping” as a result of C-C bond rotation. Further discussion about ring flipping will be included in the section on cyclohexane.
环戊烷的这种弯曲形状也称为“信封”构象。由于 C-C 键旋转,包络构象可以经历称为“环翻转”的过程。关于环翻转的进一步讨论将包含在环己烷部分。
来源于资源矩阵知识星球【仪器分析学苑】
更多内容看下边:
当你在仪器分析工作的时候:
是否曾为一份技术教程无处找到而发愁? 是否在遇到问题时候无人解答? 是否需要提升认知的时候google不到自己想要的答案? 是否面对日益激烈的分析行业无人分析? 是否想告别那些成天都是广告满天飞的行业社群? 这些问题在我们的知识星球都能解决,这是仪器分析行业(色谱质谱)专属的知识星球,一个属于▶仪器分析◀色谱质谱行业的优质付费社群! 商业的底层逻辑是什么,是最短时间得到最准确的信息,加入知识星球能帮你节约宝贵时间,让你专注学习,分享,交流!让你专注于学习、分享、交流和商业洽谈。通过最低的成本,你可以获得最大的价值。
经过近200个日日夜夜的积累,仪器分析学苑知识星球已经囊括了超过100+份产品技术文档,视频,经验分享,行业报告等等。
还能加入特定交流群得到本人和各位大佬的指导
及其丰富的书籍资源,也可以提供相关的技术咨询

随着资料逐渐增加,会员人数逐渐增多,每隔半年,星球价格会依据情况相应上浮,早加入,早享受,等待就是成本!
星球价格,不过一顿饭,但是星球却可以持续的给你提供价值,助力市场开拓,销售增长,职场提升。还能扩大行业链接,增加创富可能!
目前扫码就可以加入:
知识星球1:仪器分析学家的培养基地:仪器分析学苑

知识星球2:只需要某某元,一顿茶水钱,扫码有惊喜,医药投资、了解医药创业,投资,职业的平台,附带群资源

vx:加我微信咨询:关注公众号-我的-联系我们,扫码加
医药仪器创业方面的群聊:邀请制或者加入知识星球 总结不易,大佬肯赞赏否? END
声明:本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
垂直文章


